MTS Science White Paper – Softwave – Pain Therapy


MTS Science White Paper ©
SoftWave ™ – Pain Therapy
Research Assessment & Scientific Guide
Author: Sarah Engel (M.Sc.) Jan, 2021
CONTENT
1. Rationale…………………………………………………………………………………………………………………………………. 2
2. Introduction and Definitions……………………………………………………………………………………………………… 2
2.1. The Basic Principles of SoftWave™ Therapy…………………………………………………………………………. 2
2.2. The Basic Science of Pain…………………………………………………………………………………………………… 4
2.3. The Interface Between the Immune and Nervous Systems – Key Factors in the Pathophysiology of Pain 5
3. Pain Pathways Modulated by Shock Wave Therapy……………………………………………………………………….. 7
3.1. Nociceptive Hyperstimulation / Gate-Control Theory…………………………………………………………….. 8
3.2. Associative Pain Memory Theory…………………………………………………………………………………………. 8
3.3. Selective Degeneration and Denervation of C Fibers………………………………………………………………. 8
3.4. Altered Pain Receptor Neurotransmission……………………………………………………………………………. 9
3.5. Alleviation of Neurogenic Inflammatory Pain. Summary and Conclusions………………………………. 10
4. Summary and Conclusions……………………………………………………………………………………………………….. 11
5. Preclinical and Clinical Evidence – Abstracts……………………………………………………………………………… 12
5.1. Preclinical Evidence – Signalling Pathways Involved in the Immune-Nervous-System Interface:
Pain Resolution, Anti-Inflammatory and Pro-Angiogenic Action of SWT………………………………. 12
5.2. Clinical Evidence – Pain Relief Acute and Neuropathic Pain……………………………………………….. 23
5.3. Expert Reviews………………………………………………………………………………………………………………. 32
6. References……………………………………………………………………………………………………………………………….36

MTS_TRT_White Paper SoftWave-Pain-Therapy-Concept-2021-01-29
MTS Medical UG, Limited Liability, all rights reserved www.mts-medical.com 1
1. Rationale
An increased occurrence of pain has drastic and costly effects on the worldwide population in terms of their performance at work and daily quality of life, specifically, when moderate to severe chronic pain occurs and patients receive inadequate treatment. Many painkillers have strong side effects and are addictive. In addition, the development of tolerances leads to the use of increasingly higher doses. It is time for a global change of course in the current management of chronic pain.
For many decades, regenerative extracorporeal shock wave therapy (ESWT) has been used effectively for the treatment of acute and chronic pain in a variety of pain syndromes; For example: chronic pelvic pain, lower back pain / sacroiliac joint pain, shoulder pain, trochanteric pain, angina pectoris, intermittend claudication, etc. Whenever conservative therapy has not been effective in relieving pain and other symptoms, non-invasive ESWT has been used, yielding results of pain relief and improved function.
The precise underlying mechanism, how ESWT intervenes in the vicious cycle of (chronic) pain, is not yet fully understood. This paper describes existing hypotheses and provides the link between biological cause and medical effect of this therapy in the resolution of pain, based on the latest scientific knowledge and current findings.
2. Introduction and Definitions
2.1. The Basic Principles of SoftWave™ Therapy
SoftWaves are high-energy acoustic waves that behave much like other sound waves, except that they have much greater pressure and energy. The energy of a shock wave is released as pressure on the environment. This pressure wave builds up extremely quickly and consists of a very high positive and a minor negative part.
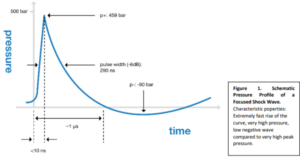
The original principle used in medicine is that, in which, the shock waves are generated by a spark plug, the electrohydraulic principle. The technology that generates the shock wave has a considerable influence on the energy distribution. SoftWave technology uses traditional electrohydraulic generation principles
MTS_TRT_White Paper-SoftWave-Pain-Therapy-Concept-2021-01-29
MTS Medical UG, Limited Liability, all rights reserved. www.mts-medical.com 2
and ensures optimum energy composition and distribution; the pressure amplitude is mainly positive with only minimal negative tensile wave energy and provides regenerative energy not only in the focal area, but in the entire acoustic field extending from the spark source.
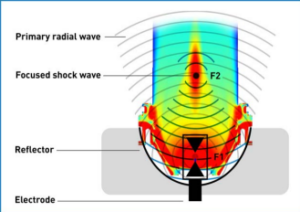
Figure 2. Eletrohydraulic Shock Wave Generation. An electrode is placed in the first focal point of a water-filled semi-ellipsoid reflector and high voltage is applied to the tips of the electrode. Thereby, an electric spark is generated between these tips and a spherical shock wave is released by the rapid vaporization of the water between the tips. The shock wave spreads out from the applicator leading to a low intensity radial primary wave, followed by a focused shockwave with focus F2 which occurs due to the reflection of the spherical wave at the reflector. The colours display imaging by a DICOM (Digital Imaging and Communications in Medicine) MATLAB (matrix laboratory) simulation of the acoustic field with SoftWave technology. Red corresponds to the area with the maximum energy.
In regenerative medicine, the physical energy of SoftWaves is applied to all kinds of tissues at a desired depth and with an adjustable energy flux density (EFD), depending on the respective indication and pathophysiology. With its unique wide focus size, SoftWave Technology delivers the highest possible amount of total biologically effective energy to the target area. This physical energy as a biological response- produces mechanical stimulation which is recognized by mechanoreceptors of the cell and transduced into to various cellular responses: The expression and release of regeneration-associated molecules, like growth factors and other signaling molecules (chemokines and cytokines), is activated. All these factors trigger intracellular signalling cascades which are implicated in processes like metabolic activation, proliferation, migration and recruiment of mesenchymal and haematopoetic progenitor cells. This action leads to reduction of inflammatory processes, as well as to improve angiogenesis and neovascularisation, resulting in tissue remodelling and regeneration.
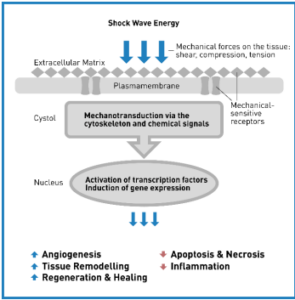 Figure 3. Mechanotransduction Mechanism – Phases of SoftWave Therapy.1. Physical Phase: Shock waves generate a positive pressure to generate absorption, reflection, scattering and transmission. 2. Chemical Phase: The mechanical stimulus leads to biochemical reaction, biomolecules are released and cell signaling pathways are activated. 3. Biological Phase: Modulation of angiogenesis, alteration of inflammatory response, bone and soft tissue healing.
Figure 3. Mechanotransduction Mechanism – Phases of SoftWave Therapy.1. Physical Phase: Shock waves generate a positive pressure to generate absorption, reflection, scattering and transmission. 2. Chemical Phase: The mechanical stimulus leads to biochemical reaction, biomolecules are released and cell signaling pathways are activated. 3. Biological Phase: Modulation of angiogenesis, alteration of inflammatory response, bone and soft tissue healing.
MTS TRT White Paper-SoftWave-Pain-Therapy-Concept-2021-01-29
MTS Medical UG, Limited Liability, all rights reserved. www.mts-medical.com
2.2. The Basic Science of Pain
Pain
As a submodality of somatic sensation, has been defined as a “complex constellation of unpleasant sensory, emotional and cognitive experiences provoked by real or perceived tissue damage and manifested by certain autonomic, psychological, and behavioral reactions”.
Nociceptors
Peripherally localized excitatory neurons preferentially sensitive to a noxious stimulus or to a stimulus that would become noxious if prolonged. Two distinct populations of afferent axons conduct impulses caused by pain, inflammation or tissue damage. The first group -the myelinated A delta and beta fibers conduct cold and well-localized pain sensations. The second group-the unmyelinated C fibers-signal pain that is poorly localized or caused by heat or mechanical stimuli (according to the Erlanger-Gasser Classification of sensory fiber types). They release glutamate as their primary neurotransmitter as well as other algesic components such as the following: prostaglandins, Substance P, calcitonin gene-related peptide (CGRP), bradykinin, and somatostatin or histamine which are important in both central synaptic signaling and efferent signaling in the skin. The fibres of nociceprtors synapse in the dorsal horn of the spinal cord where spinal modulation occurs. 2
Acute Pain
Acute (transient) high intensity stimuli yield a somatotopically limited pain sensation that resolves upon the removal of the stimuli. The encoding of the stimulus involves specific activation of subpopulations of nociceptors which are endowed with their response property by virtue of specific channels expressed on their terminals. The speed of transmission is directly correlated to the diameter of axons of sensory neurons, and whether or not they are myelinated. Most nociceptors have small diameter unmyelinated axons (C-fibers) bundled in fascicles, surrounded by Schwann cells, and support conduction velocities of 0.4-1.4 m/s). Initial fast-onset pain is mediated by A-fiber nociceptors (B and 8), whose axons are myelinated and support conduction velocities of approximately 5-30 m/s (most in the slower Ad range). This acute afferent traffic leads to activation of supraspinally projecting dorsal horn neurons; The frequency of their activation being dependent upon the frequency of afferent input and accordingly stimulus intensity.
Tissue injury
Tissue injury arising from ongoing exposure to high-intensity stimuli leads to a pain sensation continuing beyond the removal of the originating stimulus. There is, in addition, an enhanced sensitivity to otherwise modestly aversive stimuli applied to the injured tissue (hyperalgesia). Typically, such pain resolves in parallel with resolution of the injury state (healing). At the peripheral terminal, injury pain or inflammation leads to an innate immune cascade yielding release of active factors from blood, local and migrating inflammatory cells, and injured cells. These products (pro-excitatory neurohormones like cytokines or chemokines) initiate activity in C fibers, through receptors, located on the afferent terminal and sensitize these terminals. At the level of the spinal dorsal horn, ongoing afferent traffic leads to initiation of a robust facilitation of dorsal horn output.3
Development of Neuropathic / Chronic Pain
Acute pain is a warning mechanism that exists to prevent tissue damage; however, pain can outlast its protective purpose and persist beyond injury, becoming chronic. Chronic pain is maladaptive and needs addressing, as available medicines are only partially effective and cause severe side effects. Dramatic changes occur in both peripheral and central pathways resulting in an altered perception of pain characterized by Hyperalgesia, Allodynia and spontanous firing of nociceptors due to nerve damage. 4
MTS TRT White Paper-SoftWave-Pain-Therapy-Concept-2021-01-29
MTS Medical UG, Limited Liability, all rights reserved. www.mts-medical.com
Petripheral nerve damage
Leads to a pain state with persistency and components of -1. Hyperalgesia, the phenomenon where there is an enhanced sensation of pain at normal threshold stimulation. The pathophysiology is believed to arise from the sensitisation of nerves in and around the damaged area due to the release of signalling peptides and 2. Allodynia, where pain is felt on a (non-noxious) stimulus which was previously not painful. Allodynia is also observed in and around areas affected by noxious stimuli.
The peripheral and spinal mechanisms underlying this increased spontaneous nociceptor activity in tissue or peripheral nerve injury are (amongst others):
- Altered channel expression that are critical in controlling the neurotransmitter release
- Loss of inhibitory hyperpolarization of dorsal horn nociceptive neurons
- Activation and migration of non-neuronal inflammatory cells (microglia and astrocytes/T-Cells and macrphages)
- Altered expression of pro-excitatory cytokines such as the following: TNFα, bradykinin, NGF, interleukins, and catecholamines and their receptors 3
Inflammatory pain
Pain associated with tissue injury and inflammation characterized by reduced threshold and increased responsiveness.
Neurogenic inflammation
A general term used to describe the effects of the local release of inflammatory mediators such as substance P and CGRP from afferent nerve terminals.
Cytokines
Small secreted proteins released by cells that have a specific effect on the interactions and communications between cells. Cytokine is a general name; Other names include the following: lymphokine (cytokines made by lymphocytes), monokine (cytokines made by monocytes), chemokine (cytokines with chemotactic activities), and interleukin (cytokines made by one leukocyte and acting on other leukocytes). There are both pro-inflammatory cytokines and anti-inflammatory cytokines. There is significant evidence showing that certain cytokines/chemokines are involved in not only the initiation, but also the persistence of pathologic pain by directly activating nociceptive sensory neurons. Certain inflammatory cytokines are also involved in nerve-injury/inflammation-induced central sensitization, and are related to the development of contralateral hyperalgesia / allodynia. 5
2.3. The Interface Between the Immune and Nervous Systems – Key Factors in the Pathophysiology of Pain
Peripheral nerve injuries and diseases often lead to pain persisting beyond the resolution of damage, indicating an active disease-promoting process, which may result in chronic pain. This is regarded as a maladaptive mechanism resulting from neuroinflammation, that originally serves to promote regeneration and healing. Knowledge on these physiological and pathophysiological processes has accumulated over the last few decades, and has started to yield potential therapeutic targets; Key players are macrophages, T-lymphocytes, cytokines, and chemokines. In the spinal cord and brain, microglia and astrocytes are involved. Both proinflammatory and anti-inflammatory cytokines show an important role in neuropathic and other chronic pain states in humans. MicroRNAs and other noncoding RNAs have been discussed, as potential master switches, that may link nerve injury, pain, and inflammation.”
Immune cells and glia interact with neurons to alter pain sensitivity and to mediate the transition from acute to chronic pain. In response to injury, resident immune cells are activated and bloodborne immune cells are recruited to the site of injury. Immune cells not only contribute to immune protection, but also initiate the sensitization of peripheral nociceptors. Through the synthesis and release of inflammatory mediators and interactions with neurotransmitters and their receptors, the immune cells, glia, and neurons form an integrated network that coordinates immune responses and modulates the excitability of pain pathways. The immune system is also able to reduce sensitization by producing immune-derived analgesic and anti-inflammatory or proresolution agents.
Macrophages, leukocytes, mast cells, glial cells, and T lymphocytes are immune cells involved in the peripheral and central pain pathways. In response to tissue damage and nerve injury, these cells are activated and release inflammatory mediators and cytokines in the skin, peripheral nerves, dorsal root ganglia, and spinal cord. A variety of factors are released upon tissue damage which lead to the activation of nociceptors. Neurotransmitter, neuromodulators, and inflammatory mediators are released from primary afferent terminals into the spinal cord. Many of these factors are pro-inflammatory and lead to acute inflammation in the area of damage. Cytokines, tumour necrosis factor (TNF), interleukins (IL-1b, IL 6)), adenosine triphosphate (ATP), substance P, CGRP, monocyte chemotatctoc protein-1 (MCP-1/CCL2), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) are released by activated immune and glial cells (Schwann cells, microglia, satellite cells, and astrocytes). These immunological responses and the infiltration of immune cells into the CNS are involved in the pathogenesis of neuropathic and chronic pain. 7,8
Proinflammatory Cytokines are produced predominantly by activated macrophages and are involved in the up-regulation of inflammatory reactions. There is abundant evidence that certain pro-inflammatory cytokines such as IL-1ẞ, IL-6 and TNF-a are involved in the process of pathological pain. Among the many immune- or glia-derived mediators that are related to pain hypersensitivity, IL-1ẞ is a key cytokine that modulates microglia, astrocytes and neurons. IL-1ẞ was found to increase the production of substance P and prostaglandin E2 (PGE2) in a number of neuronal and glial cells. IL-6 has been shown to act as a messenger in conveying peripheral immune signals to the CNS and to contribute to neuropathic pain following a peripheral nerve injury. Blood levels become increased after inflammation. The increase in circulating IL-6 is associated with induction of cyclooxygenase-2 (COX-2) activity and Prostaglandin E2 (PGE2) release in vascular endothelial cells of the brain. Neutralization of IL-6 attenuates inflammatory hyperalgesia. TNF-a is upregulated in pain pathways after injury and secreted by immune and glial cells. 5
TNF-a (Tumor Necrose Factor Alpha) is a key inflammatory cytokine that plays a key pro-nociceptive role. It is released by stimulated macrophages, upon nerve injury, and induces peripheral nociceptor sensitization. TNF-a has been shown to play important roles in both inflammatory and neuropathic hyperalgesia and allodynia. Injection of TNFa into sciatic nerve elicits hyperalgesia and allodynia that last for days, which is associated with nerve edema, Schwann cell injury, and macrophage activation. It was shown that topically applied TNFα induced ectopic firing in C and A-delta fibers. Local TNFα also lowered the mechanical threshold of C-nociceptors and caused ongoing activity in some C nociceptors. The level of TNFa in DRG is increased after peripheral nerve injury, as well as, the two TNFa receptors, TNFR1, and TNFR2. TNFα blockade prevented or relieved neuropathic pain. 5
Anti-Inflammatory Cytokines are a series of immunoregulatory molecules that control the pro inflammatory cytokine response. Major anti-inflammatory cytokines include interleukin (IL)-1 receptor antagonist, IL-4, IL-10, IL-11, and IL-13. Leukemia inhibitory factor, interferon-alpha, IL-6, and transforming growth factor (TGF)-ẞ are categorized as either anti-inflammatory or proinflammatory cytokines, under various circumstances. Specific cytokine receptors for IL-1, TNF-α, and IL-18 also function as inhibitors for pro-inflammatory cytokines. Among all the anti-inflammatory cytokines, IL-10 is a cytokine with potent anti-inflammatory properties, repressing the expression of inflammatory cytokines, such as, TNF-α, IL-6, and IL-1 by activated macrophages. In addition, IL-10 can up-regulate endogenous anti-cytokines and down-regulate pro-inflammatory cytokine receptors. 5
Substance P’s most well-known function is as a neurotransmitter and a modulator of pain perception, by altering cellular signaling pathways. Substance P is also a key molecule in the neurogenic inflammation response, a critical interaction between the nervous system and the immune system. Additionally, the function of substance P is involved in the pathogenesis of various diseases, including but not limited to the following: cancer, diabetes, rheumatoid arthritis, myocarditis, heart failure, epilepsy, migraine, thrombosis, pruritus, depression, and anxiety.9
CGRP (Calcitonin Gene-Related Peptide) is a member of the calcitonin family of peptides. It is widely distributed in nociceptive patways in both the peripheral and the central nervous systems. Furthermore, it is a potent vasodilator. When synthesized in the dorsal horn of the spinal cord, CGRP is linked to the transmission of pain. The current literature suggests that CGRP may play a role in nociception of somatic pain conditions and proinflammatory role in chronic pain. 10
NO (Nitric Oxide) is involved in many physiological processes and several lines of evidence have indicated that NO plays a complex and diverse role in the modulation of pain. Nitric oxide is an important neurotransmitter involved in the nociceptive process and, in the dorsal horn of the spinal cord, it contributes to the development of central sensitization. On the other hand, experimental data have also demonstrated that NO inhibits nociception in the peripheral and also in the central nervous system. In addition, it has been shown that nitric oxide mediates the analgesic effect of opioids and other analgesic substances. 11
BNDF (Brain-derived Neurotrophic Fator) is a crucial neuromodulator in pain transmission, both in peripheral and central nervous system (CNS). Despite evidence of a pro-nociceptive role of BDNF, recent studies have reported contrasting results, including anti-nociceptive and anti-inflammatory activities. 12
TLRs (Toll-Like Receptors) are transmembrane protein receptors present in dendritic cells, macrophages, and glial cells. TLRs found on the Schwann cells surrounding the C fibres of primary sensory neurons have an important role in opioid tolerance and in the initiation of both inflammation and neuropathic pain. Of the 10 subtypes of TLRs in humans, the TLR2, TLR3 and TLR4 subtypes are crucial for glial activation and response in neuropathic and chronic pain mechanisms. TLR activation releases pro-inflammatory mediators such as MCP-1, ROS, NO, IL-6, IL-1a, TNF, IL-5, IL-3, IFN b, CXCL10, CCL5, inducible NO synthase, PGE2 and calcitonin gene-related peptide7
3. Pain Pathways Modulated by Shock Wave Therapy
SWT efficiently relieves acute and long-lasting chronic pain in a multiple of indications. The question remains, which basic biomolecular mechanisms and signalling pathways modulated by SWT are responsible for this effect? Based on research and clinical evidence, different aspects and multifactorial correlations are considered to be responsible for the analgesic effect of shock wave therapy.
MTS TRT White Paper-SoftWave-Pain-Therapy-Concept-2021-01-29
MTS Medical UG, Limited Liability, all rights reserved. www.mts-medical.com
3.1. Nociceptive Hyperstimulation / Gate-Control Theory
The expected mechanism is based on the interruption of high-frequent nerve impulses of peripheral nociceptors, by the intense mechanical pressure of shock wave, in the treated tissue (hyperstimulation analgesia). These intense stimulations are transmitted to the central nervous system, through the posterior column of the spinal cord, and are supposed to activate the descending inhibitory system that block a subsequent transmission of nociceptive stimuli, according to the gate-control theory. 13 The gate control theory, proposed by Melzack and Wall, states that the neural signals in the dorsal horn from the peripheral input will increase or decrease the flow of impulses to higher processing centers in the central nervous system. 2 This means that in a standard nociceptive system, for instance, the amount of pain generated by a primary nociceptive stimulus, will be reduced during and after the presentation of a second nociceptive stimulus. This is due to the activation of endogenous analgesia, i. e., the release of endorphins and other analgesic molecules. Since the analgesic effects of SWs are more prominent when the maximum energy density tolerable by the patient is applied, it is therefore reasonable to accredit analgesia to the activation of the descending inhibitory system. 14
It is also assumed that shock waves change the nature of the cell membranes, and thus, no action potential can be built up, consequently pain perception is reduced. Furthermore, shock waves change the chemical environment of the cell membranes by generating free radicals, which in turn result in pain-inhibiting chemicals in the vicinity of the cells. 15-19
3.2. Associative Pain Memory Theory
Another theory introduced by Dr. O. Wess, in 2008, was the associative model for establishing reflex functions, the formation of a pain memory. Chronic pain, for example, without underlying anatomical disorders is considered a pathological control function. An interaction between afferent sensor input and efferent motor output is postulated to form a reflex-like response. The hypothesis behind is a “malfunction” of the nervous control system due to “pathologic reflexes,” which may be conditioned by single overuse or long-term misuse of particular organs or functions. Accordingly, a circulus vitiosus of pain sensation and muscle and/ or vessel contraction is generated when pain becomes chronic. Pathological adaptation to unnatural muscular and vascular tone conditions is assumed to take place, within the nervous system, on the synaptic level by modification of the synaptic strength of large ensembles of neurons. Adaptation of synaptic threshold patterns is deemed to be the basis of memory functions in general. Consequently, successful treatment regimes must affect the pathological reflex bow and erase the particular memory, instead of modifying the organ itself. ESWT with strong and repeated stimulation of synaptic junctions may delete the pathologic memory reflex pattern selectively, with respect to the treated pain area.
However, Dr. Wess’ associative pain memory theory has been criticised with regard to the idea of seperating pain from pathology, 20
3.3. Selective Degeneration and Denervation of C Fibers
Another main hypothesis is that the analgesic effect is mediated by the selective degeneration of sensory neurons, during shock wave application, which reduces the concentration of pro-inflammatory mediators and relieves chronic pain.
To confirm this hypothesis, high-energy ESWT was applied to the ventral side of the right distal femur of rabbits. The femoral and sciatic nerves were investigated at the light and electron microscopic level after 6 weeks. ESWT caused a selective, substantial loss of unmyelinated nerve fibers, within the femoral nerve of the treated hind limb, while the sciatic nerve of the treated hind limb remained unaffected. This probably implied that a relief of chronic pain by a transient dysfunction of nerve excitability at neuromuscular junction via selective partial denervation (degeneration of acetylcholine receptor in free nerve ending) plays an important role in the effects of ESWT application on the musculoskeletal system. 14,21-23
Earlier, a Japanese group investigated the analgesic properties of shock wave application and analyzed whether it produces morphologic changes, in cutaneous nerve fibres, in rats. In normal rat skin, the epidermis is heavily innervated by nerve fibres immunoreactive for protein gene product (PGP) 9.5 and by some fibres immunoreactive for calcitonin gene-related peptide (CGRP). There was nearly complete degeneration of epidermal nerve fibres in the shock wave-treated skin, as indicated by the loss of immunoreactivity for PGP 9.5 or CGRP. Reinnervation of the epidermis occurredtwo weeks after treatment. This data shows that relief of pain, after shock wave application, to the skin results from rapid degeneration of the intracutaneous nerve fibres.” This group later demonstrated that a second application of low-energy shock waves has a cumulative effect on free nerve endings and leads to a longer lasting antinociceptive action. 25 They also showed that shock wave exposure to the footpad significantly increased the average number of neurons immunoreactive for activating transcription factor 3 (ATF3) and growth-associated phosphoprotein (GAP-43). Shockwave exposure induced injury of the sensory nerve fibers within the exposed area. They suggested that this phenomenon may be linked to the fast desensitization of the exposure area during application and that subsequent active axonal regeneration may account for the reinnervation and the amelioration of the desensitization. 14.30,20
3.4. Altered Pain Receptor Neurotransmission
The observed analgesic effect may also be given by the stimulation of the production of endogenous endorphins (substances which play a fundamental role in decreasing pain sensitivity), and by the inhibition of some specific receptors responsible for the activation of pain. The release of bioactive substances -in particular substance P and calcitonin gene-related peptide (CGRP)-released at the level of sensory nerve endings plays an important role in the maintenance of pain and chronic inflammation, 16
C fibers make synapses in the dorsal horn of the spinal cord They release substance P as neurotransmitter, which is a neuropeptide formed slowly at the synapse and is also slowly destroyed; therefore, after the start of pain stimulation, its concentration in the synaptic space increases for several seconds and lasts for a few minutes after the stimulation is ended; Thus explaining why slow chronic pain gradually increases in intensity with time and persists even after the cessation of the painful stimulus. CGRP is a marker of sensory neurons, typically involved with pain perception, and was isolated, along with substance P, in capsaicin sensitive axons. Both act on peripherals target cells such as mast cells, immune cells, and vascular smooth muscle cells, causing inflammation. This phenomenon is called neurogenic inflammation.
Studies performed on animals suggest that ESWT may have an influence on pain transmission to the brainstem, by acting on substance P and calcitonin gene gene-related peptide (CGRP) expression in the dorsal root ganglion and on neurovascular sprouting.¹
ESW application to rat femurs resulted in a short-term increase of substance P at 6 hours and 24 hours post treatment, but it was significantly decreased after 6 weeks. By contrast, extracorporeal shock wave application did not result in altered prostaglandin E release from the periosteum from the femur. Remarkably, there was a close relationship between the time course of substance P release found here and the well-known clinical time course of initial pain occurrence and subsequent pain relief after extracorporeal shock wave application to tendon diseases. Accordingly, substance P might be involved in the biologic action of extracorporeal shock wave application on tissue of the musculoskeletal system, 2?
A study in rats showed that application of shock waves to the skin decreases calcitonin gene-related peptide immunoreactivity in dorsal root ganglion neurons and suggests that relief of clinical pain may result from reduced CGRP expression in DRG neurons. 2
However, data regarding the altered expression of these pain transmitting neurohormones is controversial, as some research groups were unable to demonstrate a possible influence of low-energy ESWT on the expression of the transmitters substance P and calcitonin gene-related peptide (CGRP) in the lumbar spinal cord of the rat. Same group demonstrated no changes in the expression of endogenous opioids -met-enkephalin (MRGL) and dynorphin (Dyn)- in the spinal cord after ESWT. Therefore, the authors conclude that the endogenous opioid system is not influenced by ESWT treatment, and that it is unlikely that the application of ESWT triggers the endogenous pain control system of the rat through hyperstimulation analgesia, 29 In line with this, studies in horses and sheep failed to show differences in neuropeptide concentrations after shock wave application and substance P- and CGRP-containing nerve fibers were not disrupted. 201
3.5. Alleviation of Neurogenic Inflammatory Pain
Immune cells are significantly participating in peripheral and central pain transmission. They release inflammatory mediators that stimulate nociceptors and are therefore crucial in the development of chronic/neuropathic pain. Several studies reported that low-energy ESWT stimulates a polarity shift in the macrophage phenotype from M1 to M2. 235 This is particularly valuable for the inflamed cellular microenvironment from a standpoint of the regenerative potential of ESWT, because the macrophage expresses two major phenotypes. These are M1 and M2 and may depend on the given chemical signal. Since M1 is usually stimulated by microbial agents, it takes on a pro-inflammatory role. Conversely, the M2 macrophage is produced by the T-helper type 2 (Th2) immune response and exhibits an anti inflammatory property, typically characterized by an increase in the biosynthesis of interleukins IL-4, IL-5, IL-9, and IL-13. The type 2 response is known to be directly involved in regenerative processes, after injury and macrophages elicit their protective role, mainly by the promotion of angiogenesis via the release of cytokines and growth factors. The M2 macrophage has also shown stimulation of cell proliferation and repair through polyamine and collagen synthesis in addition to other tissue remodeling functions, releasing IL-10 and IL-4. The M1 type on the other hand displays microbicidal activity and inhibits cell proliferation, releasing the inflammatory IL-6 and tumor necrosis factor-a (TNFa) cytokines. 14
Accordingly, a clinical study in 33 patients with early osteonecrosis of the femoral head confirmed the significant reduction in pain, related to relevant biomarkers which were tested in the blood; The study revealed significant increases of serum biomarkers including, angiogenesis (NO3 and VEGF), osteogenesis (BMP-2 and osteocalcin), and regeneration (IGF) within one week to one month after the application of high dosage ESWT. At the same time, significant decreases in inflammatory cytokines (TNF-aand IL-6), pain threshold (substance P and CGRP), and tissue regeneration inhibitor (DKK-1) were also noted within one week to one month after ESWT. Therefore, it appears that ESWT is associated with systemic changes in serum biomarkers for angiogenesis, osteogenesis, anti-inflammation, pain threshold, and tissue regeneration.
4. Summary and Conclusions
There is a wealth of scientific evidence that SoftWave Therapy has the potential to modulate the immune response and rebalance the key players involved in acute and chronic pain (preclinical and clinical evidence see chapter 5.). This potential of SoftWave Therapy, to address multiple focal points of pain modulation, allows clinicians to effectively address peripheral, spinal, and supraspinal mechanisms of pain transmission.
SoftWave Therapy positively influences the activity of immune cells, has an anti-inflammatory potential, including the stimulation of mesenchymal cells and the release of factors relevant to regeneration and healing. In addition, the shock wave intervenes directly in the signal transmission of pain and has both short-term and long-term analgesic effects.
Overall, there is no common consensus on only one mechanism by which ESWT conveys its analgesic effect. Pain involves multifactorial and complex processes and the causes of pain are also Individually dependent on the nature of the underlying pathology. This is exactly the advantage of SoftWave Therapy, which intervenes at various levels of pain transmission, relieving acute pain in the short term and providing long-term relief by dissolving the actual cause through regeneration and healing.
Mechanisms underlying the effcient analgesic effect of SoftWave Therapy in acute and neuropathic persistent pain include:
- Reduced tissue inflammation and oxidative stress
- Increased angiogenesis, cell proliferation, and tissue repair
- Improved blood circulation and tissue supply
- Healing tissue by increased angiogenesis and revascularization → Re-establishment of afunctional vasculature protects against pain
- Increase of local pain inhibiting substances
- Release of trigger points, reduction in passive muscle tone and spasticity
- Modulation of neuro-immune pathways by inhibition of pro-inflammatory signalling and stimulation of anti-inflammatory signalling at the neuromimmune interface
- Influencing the neuroplasticity of the pain memory
- Altered pain-receptor neurotransmission
SoftWave Therapy is a non-invasive method to modulate the communication between neurons and immune cells and effectively treat neuropathic pain conditions. It is an outpatient procedure of short duration and can be repeated as often as needed. The therapy has no side effects and therefore has great advantages compared to systemic exposure to drugs (eg analgesics), which typically leads to considerable side effects, especially when administered over a longer period.
5. Preclinical and Clinical Evidence – Abstracts
5.1. Preclinical Evidence-Signalling Pathways Involved in the Immune-Nervous-System Interface Pain Besolution Anti-inflammatory and Pro-Angiogenic Action of SWT
Note: Reference titles written in dark blue text colour were performed with TRT/MTS SoftWave
Technology
Cross selection of thematically relevant publications:
- Shock waves promote spinal cord repair via TLR3
Spinal cord injury (SCI) remains a devastating condition with poor prognosis and very limited treatment options. Affected patients are severely restricted in their daily activities Shock wave therapy (SW has shown potent regenerative properties in bone fractures wounds, and ischemic myocardium via activation of the innate immune receptor TLR3. Here, we report on the efficacy of SWT for regeneration of SCI SWI improved motor function and decreased lesion site in WT but not Tir-/- mice via inhibition of neuronal degeneration and 16-dependent recruitment and differentiation of neuronal progenitor cells. Both SWT and TLR3 stimulation enhanced neuronal sprouting and improved neuronal survival, even in human spinal cord cultures. We identified tir3 as crucial enhancer of spinal cord regeneration in zebrafish. Our findings indicate that TLR3 signaling is involved in neuroprotection and spinal cord repair and suggest that TLR3 stimulation via SWT could become a potent regenerative treatment option.
- Motor and sensory Schwann cell phenotype commitment is diminished by extracorporeal shockwave treatment in vitro
The gold standard for peripheral nerve regeneration uses a sensory autograft to bridge a motor/sensory defect site. For motor nerves to regenerate, Schwann cells (SC) myelinate the newly grown axon. Sensory SCS have a reduced ability to produce myelin, partially explaining low success rates of autografts. This issue is masked in pre-clinical research by the excessive use of the rat sciatic nerve defect model, utilizing a mixed nerve with motor and sensory SCs. Aim of this study was to utilize extracorporeal shockwave treatment as a novel tool to influence SC phenotype. SCs were isolated from motor, sensory and mixed rat nerves and in vitro differences between them were assessed concerning initial cell number, proliferation rate, neurite outgrowth as well as ability to express myelin. We verified the inferior capacity of sensory SCs to promote neurite outgrowth and express myelin-associated proteins. Motor Schwann cells demonstrated low proliferation rates, but strongly reacted to pro-myelination stimull it is noteworthy for pre-clinical research that sciatic SCs are a strongly mixed culture, not representing one or the other. Extracorporeal shockwave treatment (ESWT), induced in motor SCs an increased proliferation profile, while sensory SCs gained the ability to promote neurite outgrowth and express myelin-associated markers. We demonstrate a strong phenotype commitment of sciatic, motor, and sensory SCs in vitro, proposing the experimental use of SCs from pure cultures to better mimic clinical situations. Furthermore we provide arguments for using ESWT on autografts to improve the regenerative capacity of sensory SCS
- Low energy extracorporeal shock wave therapy promotes BDNF expression and improves functional recovery after spinal cord injury in rats”
Low-energy extracorporeal shock wave therapy (ESWT) has been used to treat various human diseases. Previous studies have shown that low-energy ESWT promoters the release of various cell growth factors and trophic factors from the cells surrounding the target lesion. The aim of the current study was to determine whether the application of low-energy ESWT upregulates the expression of brain-derived neurotrophic factor (BDNF) and reduces neural tissue damage and functional impairment using a rat model of thoracic spinal cord contusion injury. We found that low-energy ESWT promoted BDNF expression in the damaged neural tissue. The expression of BDNF was increased in various neural cells at the lesion, Additionally, low-energy ESWT increased the area of spared white matter and the number of oligodendrocytes in the injured spinal cord compared with untreated control animals. There were more axonal fibers around the injured site after the application of low-energy ESWT than contral. Importantly, low-energy ESWT improved the locomotor functions evaluated by both the BB scale and ladder rung walking test in addition to the sensory function measured using a von Frey sest. Moreover, the electrophysiological assessment confirmed that the conductivity of the central motor pathway in the injured spinal cord was restored by low energy ESWT. These findings indicate that low energy ESWT promotes BDNF expression at the lesion site and reduces the neural tissue damage and functional Impairment following spinal cord injury. Our results support the potential application of low-energy ESWT as a novel therapeutic strategy for treating spinal cord injury.
- Inflammatory mediators are potential biomarkers for extracorporeal shockwave therapy in horses
Background: Extracorporeal shockwave therapy (ESWT) can potentially mask painful injuries in equine athletes. Tests to detect whether a horse has received ESWT prior to competition are needed. Extracorporeal shockwave therapy is known to affect inflammatory mediators in other species, and if these mediators are altered in the horse, these could serve as biomarkers of ESWT. Objectives: To test the hypothesis that a single application of ESWT will alter the circulating protein concentrations of 10 inflammatory mediators in horse plasma.
Study design: Prospective repeated measures experimental study.
Methods: Eleven healthy horses were administered a single dose of ESWT on the dorsal surface of proximal MC. Blood samples were collected at 168, 144, -120, 96, -72,-70, 68, 66, 48, 24, 6, 4, 20 h before and 2, 4, 6, 24, 48, 72, 96, 168, 336 and 504 h after ESWT. Plasma concentrations of interleukin 1 beta (IL-1), IL-1 receptor antagonist (IL-IRA), IL-2, IL-4, IL-6, IL-10, IL-15, interferon gamma OFN-y), soluble toll-like receptor 2 (STLR2) and tumour necrosis factor alpha (TNF-a) were measured to assess the effects of ESWT on these mediators.
Results: Baseline concentrations of inflammatory mediators did not change substantially during the week prior to ESWT. Plasma concentrations of five inflammatory factors changed following ESWT. IL-18 and IL 6 were significantly down-regulated (P<0.01) while TNF-a, IL-1RA and TLR2 were significantly up regulated (P<0.01). The remaining cytokines were not significantly affected by ESWT.
Main limitations: This study was performed in a small number of sedentary, healthy pasture kept horses using a single dose of ESWT applied to a single location. Additional studies are necessary to determine the effect of ESWT on inflammatory mediators in athletic horses undergoing treatment for musculoskeletal injuries.
Conclusions: Plasma concentrations of TNF-α, IL-1B, IL-1RA, IL-6 and TLR2 were significantly affected by ESWT, and deserve further investigation as possible biomarkers of ESWT.
- miR-19a-3p containing exosomes improve function of ischemic myocardium upon shock wave therapy 41
Aims: As many current approaches for heart regeneration exert unfavourable side effects, the induction of endogenous repair mechanisms in ischaemic heart disease is of particular interest. Recently, exosomes carrying angiogenic miRNAs have been described to improve heart function. However, it remains challenging to stimulate specific release of reparative exosomes in ischaemic myocardium. In the present study, we sought to test the hypothesis that the physical stimulus of shock wave therapy (SWT) causes the release of exosomes. We aimed to substantiate the pro-angiogenic impact of the released factors, to identify the nature of their cargo, and to test their efficacy in vivo supporting regeneration and recovery after myocardial ischaemia.
Methods and results: Mechanical stimulation of ischaemic muscle via SWT caused extracellular vesicle (EV) release from endothelial cells both in vitro and in vivo. Characterization of EVs via electron microscopy, nanoparticle tracking analysis and flow cytometry revealed specific exosome morphology and size with the presence of exosome markers CD9, CD81, and CD63. Exosomes exhibited angiogenic properties activating protein kinase b (Akt) and extracellular-signal regulated kinase (ERK) resulting in enhanced endothelial tube formation and proliferation. A miRNA array and transcriptome analysis via next-generation sequencing were performed to specify exosome content. miR-19a-3p was identified as responsible cargo, antimir-19a-3p antagonized angiogenic exosome effects. Exosomes and target miRNA were injected intramyocardially in mice after left anterior descending artery ligation. Exosomes resulted in improved vascularization, decreased myocardial fibrosis, and increased left ventricular ejection fraction as shown by transthoracic echocardiography.
Conclusion: The mechanical stimulus of SWT causes release of angiogenic exosomes. miR-19a-3p is the vesicular cargo responsible for the observed effects. Released exosomes induce angiogenesis, decrease myocardial fibrosis, and improve left ventricular function after myocardial ischaemia. Exosome release via SWT could develop an innovative approach for the regeneration of ischaemic myocardium. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFKB pathway in a prostatitis rat model 42
Background: This study aims to evaluate the effect of extracorporeal shock wave therapy (ESWT) on chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) and to explore the mechanism.
Methods: RWPE-2 cells were randomly divided into three groups: (a) RWPE-2 group (normal control), (b) LPS groups (lipopolysaccharide inducing inflammation) and (c) ESWT groups (LPS induced RWPE-2 treated by ESWT). After ESWT was administered, cells and supernatant were collected for enzyme-linked immunosorbent assay (ELISA) and Western blot analysis in vivo, Sprague-Dawley rats (n = 30) were randomly divided into three groups: (a) normal control group, (b) prostatitis groups, and (c) ESWT groups. Prostatitis rats were induced by 17 B-estradiol and dihydrotestosterone for 4 weeks. After ESWT, prostates of each group were collected for immunohistochemistry, Western blot analysis, and ELISA.
Results: ESWT improved prostatitis by attenuating inflammation (P<.01), ESWT downregulated the expression of cyclooxygenase 2 (COX 2) through inhibiting TLR4-NFB pathway compared with the LPS group in vitro or prostatitis group in vivo (P < .05). TRAF2 mediates ERK1/2-COX2 pathway. ESWT promotes prostate tissue recovery by stimulating vascular endothelial growth factor expression (P<.01). ESWT could suppress apoptosis in the prostate Conclusions: SWT improved CP/CPPS and reduced inflammation by degrading COX-2 in microenvironment through TLR4-NEx-inhibiting pathway. TRAFZ regulator in FRK1/2-COX-2 inhibition significantly reduced inflammation, thus suggesting ESWT may be a potential and promising treatment for CP/CPPS → Long-term functional change of cryoinjury-induced detrusor underactivity and effects of extracorporeal shock wave therapy in a rat model
Purpose: To investigate the long-term functional change of cryoinjury-induced detrusor underactivity (DU) and the therapeutic potential of repeated low-energy shock wave therapy (LESW).
Methods: Fifty-six female Sprague-Dawley rats were assigned into sham and cryoinjury of bladder with or without LESW (0.05 or 0.12 mu/mm³, 200 pulses; twice a week for 2 weeks after cryoinjury). Under halothane anesthesia, an incision was made in lower abdomen, and cryoinjury was provoked by bilateral placement of a chilled aluminum rod on the bladder filled with 1 ml saline. Measurement of contractile responses to KCI and carbachol in vitro, conscious voiding, and histological and protein changes were performed on week 1, 2, and 4 after cryoinjury.
Results: Cryoinjury of bladder induced a significant decrease in the detrusor contraction amplitude at week 1 (55.0%) and week 2 (57.2%), but the decrease in the contractile response to KCI and carbachol was only noted at week 1. At week 1, significantly increased COX-2 and TGF-B1 expression accompanied a decrease of VEGF and CGRP expression. At week 4, there was a partial recovery of voiding function and a significant increase in the Ki-67 staining. LESW treatment at higher energy level further amplified the Ki 67 staining and improved the recovery of contraction amplitude and the expression of TGF-B1 and VEGE.
Conclusions: Cryoinjury of detrusor induces DU/UAB with functional impairment lasting for up to 4 weeks, but the associated molecular changes are restored by 2 weeks LESW improved bladder wall composition, and hastened functional recovery from cryoinjury.
- Long-term Therapeutic Effects of Extracorporeal Shock Wave-Assisted Melatonin Therapy on Mononeuropathic Pain in Rats
We evaluated the ability of extracorporeal shock wave (ECSW)-assisted melatonin (Mel) therapy to offer an additional benefit for alleviating the neuropathic pain (NP) in rats. Left sciatic nerve was subjected to chronic constriction injury (CCI) to induce NP. Animals (n = 30) were randomized into group 1 (sham operated control), group 2 (CCI only), group 3 (CC)+ ECSW), group 4 (CC) + Mel) and group 5 (CCI + ECSW +Mel). By days 15, 22 and 29 after CCI, the thermal paw withdrawal latency (TPWL) and mechanical paw withdrawal threshold (MPWT) were highest in group 1, lowest in group 2, significantly higher in group 5 than in groups 3 and 4, but they showed no difference between the later two groups (all p<0.0001). The protein expressions of inflammatory (TNF-a, NF-B. MMP-9 -18), oxidative stress (NOXs-1 24. oxidized protein), apoptotic (cleaved-caspases, cleaved-PARP), DNA/mitochondrial-damaged (v H2AX/cxtosolic techrome.Cl. microglia/astrocyte activation.lox42/GFAP), and MAPKs Inhosphorylated (p):938, p-INK, p-ERKI biomarkera in dorsal root ganglia neurons (DRGs) and in spinal dorsal horn were exhibited an opposite pattern of TPW among the five groups (all p < 0.0001). Additionally, protein expressions of Nav.1.3, Nav.1.8 and Nav.1.9 in sciatic nerve exhibited an identical pattern to inflammation among the five groups (all p<0.0001). The numbers of cellular expressions of MAPKs (p ERK1/2+/peripherin cells, p-ERK1/2+/NF200+ cells and p-INK+/peripherin cells, p-INK+/NF200+ cells) and voltage-gated sodium channels (Nav.1.8+/peripherin cells, Nav.1.8+/NF200 celb, Nav.1.9+/peripherin+cells, Nav.1.9+/NF200+cells) in small and large DRGS displayed an identical pattern to inflammation among the five groups (all p<0.0001). In conclusion, the synergistic effect of combined ECSW-Mel therapy is superior to either one alone for long-term improvement of mononeuropathic pain induced by CCI in rats.
- Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats
The effect of low energy shock wave (LESW) therapy on the changes of inflammatory molecules and pain reaction was studied in a capsaicin (10 mM, 0.1 cc) induced prostatitis model in rats. Intraprostatic capsaicin injection induced a pain reaction, including closing of the eyes, hypolocomotion, and tactile allodynia, which effects were ameliorated by LESW treatment. LESW therapy (2Hz, energy flux density of 0.12 ml/mm2) at 200 and 300 shocks significantly decreased capsaicin-induced inflammatory reactions, reflected by a reduction of tissue edema and inflammatory cells, COX-2 and TNF-a stained positive cells, however, the therapeutic effects were not observed at 100 shocks treated group. Capsaicin-induced IL 18, COX-2, IL-6, caspase-1, and NGF upregulation on day 3 and 7, while NALP1 and TNF-a upregulation was observed on day 7. LESW significantly suppressed the expression of IL-18, COX-2, caspase-1, NGF on day 3 and IL-18. TNF-. COX-2, NAIP1, aspase-1. NGF expression on day 7 in a dose-dependent fashion LESW has no significant effect on IL-6 expression, intraprostatic capsaicin injection activates inflammatory molecules and induces prostatic pain and hypersensitivity, which effects were suppressed by LESW. These findings might be the potential mechanisms of LESW therapy for nonbacterial prostatitis in humans.
- Shock Wave Treatment After Hindlimb ischaemia Results in Increased Perfusion and M2 Macrophage Presence
Shock wave therapy (SWT) has been shown to induce angiogenesis in ischaemic muscle. However, the mechanism of action remains unknown, Macrophages are crucial for angiogenic responses after ischaemic injury. The M2 macrophage subset enables tissue repair and induces angiogenesis. It was hypothesized that the angiogenic effects of SWT are at least partly caused by enhanced macrophage recruitment. C57BL/6 mice were subjected to hind limb ischaemia with subsequent SWT or sham treatment. Muscles were analysed via immunofluorescence staining, reverse-transcription polymerase chain reaction and western blot. Gene expression and proteins involved in macrophage recruitment were analysed and tissue sections were stained for macrophages, including subsets, capillaries and arterioles. Laser Doppler perfusion imaging was performed to assess functional outcome. Treated muscles showed increased expression of the pivotal macrophage recruiting factor monocyte chemotactic protein 1 (MCP-1). Higher levels of macrophage marker CD14 were found. Increased numbers of macrophages after SWT could be confirmed by immunofluorescence staining. The expression of the M2 polarization promoting chemokine interleukin 13 was significantly elevated in the treatment group. Elevated mRNA expression of the M2 scavenger receptor CD163 was found after SWT. Immunofluorescence staining confirmed increased numbers of M2 macrophages after treatment. It was found that SWT resulted in higher number of capillaries and arterioles. Assessment of functional outcome revealed significantly improved limb perfusion in treated animals. Shock wave therapy causes increased macrophage recruitment and enhanced polarization towards reparative M2 macrophages in ischaemic muscle resulting in angiogenesis and improved limb perfusion and therefore represents a promising new treatment option for the treatment of ischaemic heart disease.
- Extracorporeal shockwave against inflammation mediated by GPR120 receptor cyclophosphamide induced rat cystitis model 46
Background: We tested the hypothesis that extracorporeal shockwave treatment (ESWT) can abolish inflammation and restore urothelial barrier integrity in acute interstitial cystitis by upregulating the fatty acid receptor GPR120.
Methods: A total of 30 female Sprague-Dawley rats were categorized into five groups: (1) sham-operated rats (SC); (2) rats treated with ESWT (SC + ESWT); (3) rats with bladder irritation using 150 mg/kg cyclophosphamide through intraperitoneal injection; (4) cyclophosphamide rats treated with ESWT (cyclophosphamide+ESWT); (5) cyclophosphamide rats treated with GPR120 agonist (cyclophosphamide+GW9508).
Results: On Day 3, urine and bladder specimens were collected for biochemical, histopathological, immunological, and immunoblotting analysis. Following stimulation with cyclophosphamide, the inhibition of the elevated levels of TAK1/NF-kB and phospho-TAK1/NF-kB by ESWT and GPR120 agonists in RT4 cells was associated with a suppression of NF-κB translocation from the cytosol to the nucleus.
Accordingly, this anti-inflammatory effect was abolished by GPR120 antagonist and knockdown of GPR120. Histologically, bladder inflammation in cyclophosphamide-treated rats was suppressed by GW9508 or ESWT. Masson’s trichrome and Sirius red staining revealed that cyclophosphamide treatment enhanced synthesis of extracellular matrix in rats that was reversed by GW9508 or ESWT. Upregulated pro-inflammatory mediators and cytokines in the cyclophosphamide-treated rats were also suppressed in the GW9508-or ESWT-treated rats. The significantly increased inflammatory cell infiltration as well as the impaired urothelial integrity of the bladder after cyclophosphamide treatment were reversed by treatment with GW9508 or ESWT.
Conclusions: These findings suggest that GPR120, the sensing receptor for ESWT, may be useful in the treatment of interstitial cystitis by inhibiting inflammatory response in bladder cells.
- Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signalling Pathway 47
Low-intensity extracorporeal shock wave therapy (Li-ESWT) is used in the treatment of erectile dysfunction, but its mechanisms are not well understood. Previously, we found that Li-ESWT increased the expression of brain-derived neurotrophic factor (BDNF). Here we assessed the underlying signaling pathways in Schwann cells in vitro and in penis tissue in vivo after nerve injury. The result indicated that BDNF were significantly increased by the Li-ESWT after nerve injury, as well as the expression of BDNF in Schwann cells (SCs, RT4-D6P2T) in vitro. Li-ESWT activated the protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK) pathway by increasing the phosphorylation levels of PERK and eukaryotic initiation factor 2a (eIF2α), and enhanced activating transcription factor 4 (ATF4) in an energy-dependent manner. In addition, GSK2656157-an inhibitor of PERK-effectively inhibited the effect of Li-ESWT on the phosphorylation of PERK, eIF2α, and the expression of ATF4. Furthermore, silencing ATF4 dramatically attenuated the effect of Li-ESWT on the expression of BDNF, but had no effect on hypoxia-inducible factor (HIF)1α or glial cell-derived neurotrophic factor (GDNF) in Schwann cells. In conclusion, our findings shed new light on the underlying mechanisms by which Li-ESWT may stimulate the expression of BDNF through activation of PERK/ATF4 signaling pathway. This information may help to refine the use of Li-ESWT to further improve its clinical efficacy.
- Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model 48
Aims: Low energy shock wave (LESW) is known to facilitate tissue regeneration with analgesic and anti-inflammatory effects. We examined the effects of LESW on the expression of inflammatory molecules, pain behavior, and bladder function in a rat cystitis model.
Methods: Control and experimental animals were injected with saline or cyclophosphamide (CYP; 75 mg/kg intraperitoneally) on day 1 and 4. After lower midline incision, the bladders were exposed to LESW (300 pulses, 0.12 mJ/mm2) or sham operation on day 2. In study 1 (N = 12, 4 for each group), the nociceptive effects of CYP were evaluated for 30 min by behavioral assessment on day 4 one hour after CYP injection. In study 2 (N = 21, 7 for each group), continuous cystometry (CMG) was performed on day 8. The bladder was harvested after behavioral assessment or CMG for histology and Western blotting.
Results: CYP-induced upregulation of COX2 and IL6 expression, caused pain behavior (eye closing and hypolocomotion), and bladder inflammation was noted on days 4 and 8 along with bladder hyperactivity. LESW treatment reduced pain behavior and downregulated the NGF expression (33.3%, P < 0.05) on day 4 and IL6 (40.9%, P < 0.05). LESW treatment suppressed bladder overactivity (intercontraction interval 77.8% increase, P < 0.05) by decreasing inflammation and COX2 (38.6%, P < 0.05) expression and NGF expression (25.2%, P = 0.0812).
Conclusions: CYP-induced bladder pain, inflammation, and overactivity involves activation of IL6, NGF, and COX2 expression. These changes are suppressed by LESW, indicating it as a potential candidate for relieving bladder inflammatory conditions and overactivity.
- Toll-like Receptor 3 Signalling Mediates Response upon Shock Wave Treatment of Ischaemic Muscle 49
Aims: Shock wave therapy (SWT) represents a clinically widely used angiogenic and thus regenerative approach for the treatment of ischaemic heart or limb disease. Despite promising results in preclinical and clinical trials, the exact mechanism of action remains unknown. Toll-like receptor 3, which is part of the innate immunity, is activated by binding double-stranded (ds) RNA. It plays a key role in inflammation, a process that is needed also for angiogenesis. We hypothesize that SWT causes cellular cavitation without damaging the target cells, thus liberating cytoplasmic RNA that in turn activates TLR3.
Methods and results: SWT induces TLR3 and IFN-β1 gene expression as well as RNA liberation from endothelial cells in a time-dependant manner. Conditioned medium from SWT-treated HUVECs induced TLR3 signalling in reporter cells. The response was lost when the medium was treated with RNase III to abolish dsRNAs or when TLR3 was silenced using siRNAs. In a mouse hind limb ischaemia model using wt and TLR3(-/-) mice (n = 6), SWT induced angiogenesis and arteriogenesis only in wt animals. These effects were accompanied by improved blood perfusion of treated limbs. Analysis of main molecules of the TLR3 pathways confirmed TLR3 signalling in vivo following SWT.
Conclusion: Our data reveal a central role of the innate immune system, namely Toll-like receptor 3, to mediate angiogenesis upon release of cytoplasmic RNAs by mechanotransduction of SWT.
- The Influence of Shockwave Therapy on Orthodontic Tooth Movement Induced in the Rat 50
Shockwave therapy is used in medicine due to its ability to stimulate healing processes. The application of orthodontic force evokes an inflammatory reaction resulting in tooth movement. Shockwave therapy might have an effect on both inflammatory and periodonal ligament cytokine profiles. Our aim was to evaluate the fluctuations of different inflammatory cytokines after orthodontic force induction with and without shockwave therapy. An orthodontic appliance was applied between the rats’ molars and incisors.
In conjunction with the commencement of orthodontic force, the rats were treated with a single episode of 1000 shock waves and the gingival crevicular fluid was collected for 3 days. The expression and concentration of different cytokines was evaluated by a commercial 4-multiplex fluorescent bead-based immunoassay. The level of all cytokines displayed a similar trend in both shockwave-treated and untreated groups; the concentration peaked on the first day and declined thereafter. In all cases, however, the cytokine levels were smaller in the shockwave-treated than in untreated animals; a significant difference was found for sRANKL and borderline difference for IL-6 on Day 1. We conclude that shockwave therapy during the induction of orthodontic tooth movement influences the expression of inflammatory cytokines.
- Effects of Shock Waves on Expression of IL-6, IL-8, MCP and TNF-α Expression by Human Periodontal Ligament Fibroblasts: an In Vitro Study 51
Background: Extracorporeal shock wave therapy (ESWT) can modulate cell behavior through mechanical information transduction. Human periodontal ligament fibroblasts (hPDLF) are sensible to mechanical stimulus and can express pro-inflammatory molecules in response. The aim of this study was to evaluate the impacts of shock waves on interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP-1), and tumor necrosis factor-alpha (TNF-α) expression by hPDLF.
Material/methods: After being treated by shock waves with different parameters (100-500 times, 0.05-0.19 mJ/mm(2)), cell viability was tested using CCK-8. IL-6, IL-8, MCP-1, and TNF-α gene expression was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) and IL-6 and IL-8 protein was measured by enzyme-linked immunosorbent assay (ELISA) at different time points.
Results: Shock waves with the parameters used in this study had no significant effects on the viability of hPDLF. A statistical inhibition of IL-6, IL-8, MCP-1, and TNF-α expression during the first few hours was observed (P<0.05). Expression of IL-8 was significantly elevated in the group receiving the most pulses of shock wave (500 times) after 4 h (P<0.05). At 8 h and 24 h, all treated groups demonstrated significantly enhanced IL-6 expression (P<0.05). TNF-α expression in the groups receiving more shock pulses (300, 500 times) or the highest energy shock treatment (0.19 mJ/mm(2)) was statistically decreased (P<0.05) at 24 h.
Conclusions: Under the condition of this study, a shock wave with energy density no higher than 0.19 mJ/mm(2) and pulses no more than 500 times elicited no negative effects on cell viability of hPDLF. After a uniform initial inhibition impact on expression of inflammatory mediators, a shock wave could cause dose-related up-regulation of IL-6 and IL-8 and down-regulation of TNF-α.
- Shock Wave Treatment Protects From Neuronal Degeneration via a Toll-Like Receptor 3 Dependent Mechanism: Implications of a First-Ever Causal Treatment for Ischemic Spinal Cord Injury 52
Background: Paraplegia following spinal cord ischemia represents a devastating complication of both aortic surgery and endovascular aortic repair. Shock wave treatment was shown to induce angiogenesis and regeneration in ischemic tissue by modulation of early inflammatory response via Toll-like receptor (TLR) 3 signaling. In preclinical and clinical studies, shock wave treatment had a favorable effect on ischemic myocardium. We hypothesized that shock wave treatment also may have a beneficial effect on spinal cord ischemia.
Methods and results: A spinal cord ischemia model in mice and spinal slice cultures ex vivo were performed. Treatment groups received immediate shock wave therapy, which resulted in decreased neuronal degeneration and improved motor function. In spinal slice cultures, the activation of TLR3 could be observed. Shock wave effects were abolished in spinal slice cultures from TLR3(-/-) mice, whereas the effect was still present in TLR4(-/-) mice. TLR4 protein was found to be downregulated parallel to TLR3 signaling. Shock wave-treated animals showed significantly better functional outcome and survival. The protective effect on neurons could be reproduced in human spinal slices.
Conclusions: Shock wave treatment protects from neuronal degeneration via TLR3 signaling and subsequent TLR4 downregulation. Consequently, it represents a promising treatment option for the devastating complication of spinal cord ischemia after aortic repair.
- Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling 53
Introduction: Extracorporeal Shock Wave Therapy (ESWT) is broadly used as a non-surgical therapy in various diseases for its pro-angiogenic and anti-inflammatory effects. However, the molecular mechanisms translating tissue exposure to shock waves (SW) in a biological response with potential therapeutic activity are largely unknown. As macrophages take part in both the onset and amplification of the inflammatory response, and well in its resolution, we investigated the effect of SW on their biology.
Methods: Human monocyte-derived macrophages were polarized to classic (M1) pro inflammatory macrophages or alternative (M2) anti-inflammatory macrophages and exposed to SW ad different intensities. Expression levels of marker genes of macrophage activation were measured by qPCR at different time points.
Results: SW did not induce activation of resting macrophages at any energy level used. Conversely, when used at low energy SW caused a significant inhibition of some M1 marker genes (CD80, COX2, CCL5) in M1 macrophages and a significant synergistic effect for some M2 marker genes (ALOX15, MRC1, CCL18) in M2 macrophages. SW also affected cytokine and chemokine production, inducing in particular a significant increase in IL-10 and reduction in IL-1β production.
Conclusions: Macrophage exposure to low energy SW dampens the induction of the pro-inflammatory profile characterizing M1 macrophages and promotes the acquisition of an anti inflammatory profile synergizing with macrophage alternative activation.
- Alteration of Inflammatory Response by Shock Wave Therapy Leads to Reduced Calcification of Decellularized Aortic Xenografts in Mice 54
Objectives: Tissue-engineered xenografts represent a promising treatment option in heart valve disease. However, inflammatory response leading to graft failure and incomplete in vitro repopulation with recipient cells remain challenging. Shock waves (SWs) were shown to modulate inflammation and to enhance re-epithelialization. We therefore aimed to investigate whether SWs could serve as a feasible adjunct to tissue engineering.
Methods: Porcine aortic pieces were decellularized using sodium deoxycholate and sodium dodecylsulphate and implanted subcutaneously into C57BL/6 mice (n = 6 per group). The treatment (shock wave therapy, SWT) group received SWs (0.1 mJ/mm(2), 500 impulses, 5 Hz) for modulation of inflammatory response directly after implantation; control animals remained untreated (CTR). Grafts were harvested 72 h and 3 weeks after implantation and analysed for inflammatory cytokines, macrophage infiltration and polarization, osteoclastic activity and calcification. Transmission electron microscopy (TEM) was performed. Endothelial cells (ECs) were treated with SWs and analysed for macrophage regulatory cytokines. In an ex vivo experimental set-up, decellularized porcine aortic valve conduits were reseeded with ECs with and without SWT (0.1 mJ/mm(2), 300 impulses, 3 Hz), fibroblasts as well as peripheral blood mononuclear cells (all human) and tested in a pulsatile flow perfusion system for cell coverage.
Results: Treated ECs showed an increase of macrophage migration inhibitory factor and macrophage inflammatory protein 1β, whereas CD40 ligand and complement component C5/C5a were decreased. Subcutaneously implanted grafts showed increased mRNA levels of tumour necrosis factor α and interleukin 6 in the treatment group. Enhanced repopulation with recipient cells could be observed after SWT. Augmented macrophage infiltration and increased polarization towards M2 macrophages was observed in treated animals. Enhanced recruitment of osteoclastic cells in proximity to calcified tissue was found after SWT. Consequently, SWT resulted in decreased areas of calcification in treated animals. The reseeding experiment revealed that fibroblasts showed the best coverage compared with other cell types.
Moreover, SW-treated ECs exhibited enhanced repopulation compared with untreated controls.
Conclusions: SWs reduce the calcification of subcutaneously implanted decellularized xenografts via the modulation of the acute macrophage-mediated inflammatory response and improves the in vitro repopulation of decellularized grafts. It may therefore serve as a feasible adjunct to heart valve tissue engineering.
- Selective loss of unmyelinated nerve fibers after extracorporeal shockwave application to the musculoskeletal system 21
Application of extracorporeal shockwaves (ESW) to the musculoskeletal system may induce long-term analgesia in the treatment of chronic tendinopathies of the shoulder, heel and elbow.
However, the molecular and cellular mechanisms behind this phenomenon are largely unknown. Here we tested the hypothesis that long-term analgesia caused by ESW is due to selective loss of nerve fibers in peripheral nerves. To test this hypothesis in vivo, high-energy ESW were applied to the ventral side of the right distal femur of rabbits. After 6 weeks, the femoral and sciatic nerves were investigated at the light and electron microscopic level. Application of ESW resulted in a selective, substantial loss of unmyelinated nerve fibers within the femoral nerve of the treated hind limb, whereas the sciatic nerve of the treated hind limb remained unaffected. These data might indicate that alleviation of chronic pain by selective partial denervation may play an important role in the effects of clinical ESW application to the musculoskeletal system.
- Extracorporeal shockwave application to the distal femur of rabbits diminishes the number of neurons immunoreactive for substance P in dorsal root ganglia L5 22
Application of extracorporeal shockwaves to the musculoskeletal system can induce long-term analgesia in the treatment of chronic painful diseases such as calcifying tendonitis of the shoulder, tennis elbow and chronic plantar fasciitis. However, the molecular and cellular mechanisms underlying this phenomenon are largely unknown. Recently it was shown that application of extracorporeal shockwaves to the distal femur of rabbits can lead to reduced concentration of substance P in the shockwaves’ focal zone. In the present study we investigated the impact of extracorporeal shockwaves on the production of substance P within dorsal root ganglia in vivo. High-energy shockwaves were applied to the ventral side of the right distal femur of rabbits. After six weeks, the dorsal root ganglia L5 to L7 were investigated with high-precision design-based stereology. The application of extracorporeal shockwaves caused a statistically significant decrease in the mean number of neurons immunoreactive for substance P within the dorsal root ganglion L5 of the treated side compared with the untreated side, without affecting the total number of neurons within this dorsal root ganglion. No effect was observed in the dorsal root ganglia L6 and L7, respectively. These data might further contribute to our understanding of the molecular and cellular mechanisms in the induction of long-term analgesia by extracorporeal shockwave application to the musculoskeletal system.
- Immunohistochemical evaluation of substance P and calcitonin gene-related peptide in skin and periosteum after extracorporeal shock wave therapy and radial pressure wave therapy in sheep 30
Objective: To evaluate the effect of focused extracorporeal shock wave therapy (ESWT) and radial pressure wave therapy (RPWT) on immunohistochemical staining for substance P and calcitonin gene-related peptide (CGRP) in the skin and periosteum of sheep.
Animals: 36 sheep.
Procedures: All 4 limbs of 36 sheep were treated with ESWT, RPWT, or a sham treatment. For 14 days after treatment, at least 2 sheep were euthanized daily and tissue was harvested for histologic evaluation of nerves via staining for substance P and CGRP in the skin and periosteum.
Results: No effects of ESWT or RPWT were observed on the number of nerves with stain uptake for substance P or CGRP in the skin or periosteum.
Conclusions and clinical relevance: Substance P- and CGRP-containing nerve fibers are not disrupted by EWST or RPWT. Further studies are needed to identify the mechanism of analgesia observed in association with these treatment modalities.
- Evaluation of analgesia resulting from extracorporeal shock wave therapy and radial pressure wave therapy in the limbs of horses and sheep 31
Objective: To identify the duration and potential mechanisms of analgesia following extracorporeal shock wave therapy (ESWT) and radial pressure wave therapy (RPWT) in limbs of horses and sheep.
Animals: 6 horses and 30 sheep.
Procedure: An electrical stimulus was used to identify the nociceptive threshold for each horse daily for 3 days before treatment (baseline) with ESWT or RPWT, 8 hours after treatment, and at 24-hour intervals for 7 days after treatment. Testing was conducted for the treatment field (midmetacarpus or midmetatarsus) and nerve field (medial and lateral forelimb heel bulbs) distal to a treatment site that included the nerve on the abaxial surface of the proximal sesamoid bone. All 4 limbs of 30 sheep were treated with ESWT, RPWT, or a sham treatment. Two sheep were euthanatized daily and tissue harvested for histologic evaluation of nerves, and concentrations of substance P and calcitonin gene-related peptide were measured in the skin and periosteum.
Results: Values did not differ significantly between baseline and after treatment for the treatment field or nerve field sensation. There was a large difference in the slope when data for horses were plotted for the first 3 days after treatment, compared with the slope for days 4 to 7 after treatment. No differences were found in neuropeptide concentrations after treatment of the sheep, but there was an inflammatory response in the treated nerves.
Conclusions and clinical relevance: A small cutaneous analgesic effect may exist at the treatment site for approximately 3 days after ESWT or RPWT in horses.
- Application of shock waves to rat skin decreases calcitonin gene-related peptide immunoreactivity in dorsal root ganglion neurons 28
There have been several reports on the use of extracorporeal shock waves in the treatment of pseudarthrosis, calcifying tendinitis, and tendinopathies of the elbow. However, the pathomechanism of pain relief has not been clarified. To investigate the analgesic properties of shock wave application, we analyzed changes in calcitonin gene-related peptide (CGRP)-immunoreactive (ir) dorsal root ganglion (DRG) neurons. In the nontreated group, fluorogold-labeled dorsal root ganglion neurons innervating the most middle foot pad of hind paw were distributed in the L4 and L5 dorsal root ganglia. Of these neurons, 61% were CGRP-ir. However, in the shock wave-treated group, the percentage of FG-labeled CGRP-ir DRG neurons decreased to 18%. These data show that relief of clinical pain after shock wave application may result from reduced CGRP expression in DRG neurons. Absence of spinal response to extracorporeal shock waves on the endogenous opioid systems in the rat 55 Extracorporeal shock wave therapy (ESWT) seems to be a new therapeutic strategy for chronic pain due to tendopathies. Neurophysiological mechanisms of action for pain relief following ESWT are still unknown. The aim of this study was to investigate if the analgesic effect of ESWT is caused by modulation of the endogenous spinal opioid system. Rats were treated with two different energy flux densities (0.04 and 0.11mJ/mm(2)) and immunohistochemical analysis of met-enkephalin (MRGL) and dynorphin (Dyn) was performed at 4 or 72 h after ESWT. ESWT had no modulatory influence on the expression of the spinal opioid systems. Different energy doses or repetitive treatment did not alter MRGL or Dyn immunoreactivity in the spinal cord. Furthermore, a delayed effect of ESWT at 72 h after treatment was not detectable. We conclude from these findings that the analgesic effects of ESWT treatment are not supported by endogenous opioids.
- No influence of low-energy extracorporeal shock wave therapy (ESWT) on spinal nociceptive systems 29
The analgesic effects of high-energy extracorporeal shock wave therapy (ESWT) were discovered by chance during its application for urolithiasis and for bone pseudarthrosis. Despite the extensive use of ESWT, the mechanisms of its antinociceptive effects are still unclear. A gate control mechanism and other antinociceptive mechanisms have been postulated. The aim of this study was to investigate the possible influence of low-energy ESWT on the expression of the transmitters substance P (SP) and calcitonin gene-related peptide (CGRP) in the lumbar spinal cord of the rat. Immunohistochemical analysis of the expression of the neuropeptides CGRP and SP was performed in rats treated either once with 1000 impulses or three times with 1000 impulses, withtwo different energy flux densities being used (0.043 and 0.11 mJ/mm2). The animals were killed either 4 or 72 h after the ESWT. No regulatory effect of ESWT on the expression of SP or CGRP in the dorsal horns was found. Because the application of ESWT showed no significant changes in the sensory system, it is unlikely that the application of ESWT triggers the endogenous pain control system of the rat through hyperstimulation analgesia. Furthermore, these results show that low-energy ESWT had no side effects on the rat spinal cord.
- Unchanged c-Fos expression after extracorporeal shock wave therapy: an experimental investigation in rats 56
Background: Despite the extensive use of extracorporeal shock wave therapy (ESWT) in the therapy of chronic tendopathies, the biological mechanisms of its antinociceptive effects are still unclear.
Methods: In this study we addressed the question of whether the clinically described, long-lasting effect of ESWT is mediated by changes in the activity of spinal cord neurones. As a marker for neuronal activity which is also able to interfere with the molecular expression pattern of neurones, the expression of the inducible transcription factor c-Fos was analysed in the animal model of the rat. Despite application of different energy levels, the analysis of c-Fos protein expression at an early (4 h) and late (72 h) time point after ESWT revealed no visible changes in immunoreactivity in the dorsal horn of the spinal cord. Additionally, there was no change in c-Fos protein and its gene expression c-Fos mRNA in the treatment area of the paw.
Results and conclusion: We conclude that ESWT with an energy flux dose up to 0.33 mJ/mm(2)does not modify neuronal activity. Since the application of ESWT showed no significant changes in the immunoreactivity of c-Fos, it is therefore unlikely that ESWT triggers stimulation produced analgesia via activation of peripheral nerves.
5.2. Clinical Evidence – Pain Relief Acute and Neuropathic Pain
Note: Although difficult to measure, due to ist multifaceted and subjective nature, pain is the primary endpoint for most investigations. In the course of pain management and analysis special pain rating scales and pain questionnaires are used.
Note: Reference titles written in dark blue text colour were performed with TRT/MTS SoftWave™ Technology.
Cross selection of thematically relevant publications:
- Efficacy of broad-focused Medium-Intensity Extracorporeal Shock Wave Therapy (MI-ESWT) for Plantar Fasciitis 57
Weill Medical College of Cornell University, New York, NY
Extracorporeal shock wave therapy (ESWT) is a promising treatment for plantar fasciitis (PF), however treatment results have varied due to inconsistencies among types of shock waves treatment and devices used. This retrospective chart review includes patients who underwent EWST using the OrthoGold 100™ shock wave device (MTS, Konstanz, Germany) for PF between January, 2013 and September, 2018. There were 108 patients (119 heels) identified, with a mean age of 51.7 ± 16.5 (Range 21-83) years. Patients were treated weekly for 3 weeks, with 2000 impulses per session at an energy flux density (EFD) between 0.10 and 0.17 mJ/mm 2. Mean follow-up duration was 11.5 ± 9.7 (Range 3-51) months. Mean pre-ESWT pain visual assessment scale (VAS) improved from 6.7 ± 1.7 to 2.6 ± 2.7 (p< 0.001). The Foot and Ankle Outcome Score (FAOS) subscales: pain, function of daily living, function of sports and recreational activities and quality of life domains improved from 53.7 ± 14.9 to 75.7 ± 16.7 ( p< 0.001), from 38 ± 15.2 to 71.8 ± 23 ( p< 0.001), from 55.8 ± 16.4 to 71.4 ± 18 ( p< 0.001), from 42.4 ± 21.5 to 59.4 ± 20.3 ( p< 0.001) and from 44.9 ± 16.4 to 69 ± 23.9 ( p< 0.001), respectively. Eighty-eight patients (81.5%) were satisfied with the procedure at final follow-up. Treatment of plantar fasciitis with broad-focused shock waves was well tolerated and led to significant pain reduction, functional improvement and patient satisfaction.
- The Effects Of Extracorporeal Shock Wave Therapy On Pain, Disability And Life Quality Of Chronic Low Back Pain Patients 58
Background: Low back pain is the most common form of pain related to the musculoskeletal system disorders. ESWT has been suggested as a new treatment modality in CLBP and its effectiveness has been investigated in a small number of studies.
Objective: The aim of this study is to investigate the effect of Extracorporeal Shockwave Therapy (ESWT) on pain, functional status, and quality of life compared to placebo in chronic low back pain (CLBP) patients.
Methods/design: Prospective, randomized, placebo-controlled, double-blind study.
Setting: The study occurred at the University Of Health Sciences, Bursa Yuksek Ihtisas Training and Research Hospital, Department of Physical Medicine and Rehabilitation (Bursa, Turkey).
Participants: Participants were 45 patients with CLBP.
Interventions: Participants were randomized into 2 groups. Group 1 (n = 25) received ESWT and Group 2 (n = 20) received placebo ESWT.
Primary outcome measures: The patients were assessed by using Numerical Rating Scale (NRS), Oswestry Disability Index (ODI), Hospital Anxiety and Depression Scale (HADS), Short form 36 (SF-36). The data were obtained before treatment (W0), at sixth (W6) and twelfth week (W12).
Results: In Group 1, statistically significant improvement was found in all parameters of rest and movement NRS, ODI, HADS and SF-36 except for emotional role at both W6 and W12 compared to W0(P < .05). Comparison of the difference scores of the two groups showed significantly superior improvement in Group 1 for all parameters at both W6 and W12 (P < .05).
Conclusions: The results of our study have shown that ESWT had a statistically significant superiority over placebo for improvement in the parameters of pain, disability, depression, anxiety, and quality of life in the patients with CLBP.
- Retrospective Chart Review of Treatment Outcome Following Low-Intensity Shockwave Therapy for the Treatment of Vestibulodynia with Urogold100 59
San Diego Sexual Medicine, APC.
Introduction: Genital pain disorders have devastating effects on a woman’s quality of life, including social isolation. These disorders occur with high prevalence; more than 1/3 of women report pain during sexual activity, placing a significant financial burden on women and the healthcare system. Multiple medical treatments for dyspareunia are available to improve quality of life and decrease pain, however many are invasive, involving pharmacotherapy/hormone therapy/needle insertion/surgery, and are associated with significant morbidity. Low intensity shockwave therapy (LiSWT) is a non-invasive, non-pharmacologic, non-hormonal, non-surgical, low morbidity treatment strategy. FDA-cleared for pain amelioration in the
Click here to read more.
