Enhancing recruitment of endothelial progenitor cells

Background— Stem and progenitor cell therapy is a novel approach to improve neovascularization and function of ischemic tissue. Enhanced tissue expression of chemoattractant factors such as stromal cell–derived factor 1 and vascular endothelial growth factor is crucial for the recruitment of circulating endothelial progenitor cells (EPCs) during acute ischemia. In chronic ischemia, however, expression of these chemoattractants is less pronounced, which results in insufficient EPC recruitment into the target tissue. Therefore, we investigated the effect of targeted extracorporeal shock wave (SW) application in order to facilitate EPC recruitment into nonischemic and chronic ischemic tissue.
Methods and Results— Hind limb adductor muscles of nude rats were treated with 500, 1000, and 2000 impulses of focused low-energy SW (flux density level: 0.05 mJ/mm2). Twenty-four hours later, mRNA expression of the chemoattractant stromal cell–derived factor 1 was significantly increased with 1000 impulses (stromal cell–derived factor 1/GAPDH: 0.95±0.09) and 2000 impulses (stromal cell–derived factor 1/GAPDH: 1.17±0.24; both P<0.05 versus untreated). Histologically, the number of vascular endothelial growth factor–positive endothelial cells per myocyte was significantly increased with 2000 impulses (0.24±0.05 versus 0.09±0.02; P<0.01). This preconditioning effect resulted in significantly enhanced recruitment and homing of EPCs that were intravenously infused 24 hours after SW treatment (P<0.05). In a rat model of chronic hind limb ischemia, SW-facilitated EPC treatment resulted in a significant increase in relative blood flow recovery as assessed by laser Doppler imaging (P<0.05).
Conclusions— Preconditioning of both nonischemic and chronic ischemic tissue with low-energy SW improves recruitment of circulating EPCs via enhanced expression of chemoattractant factors. Thus, SW-facilitated cell therapy may improve the efficacy of EPC treatment in patients with chronic ischemia.Stem or progenitor cell therapy is a novel approach to improve functional recovery of ischemic tissue. Experimental studies and, more recently, phase II and III clinical trials documented that administration of bone marrow–derived or peripheral blood–derived progenitor cells increased neovascularization and reduced infarct size and scar formation after myocardial infarction.1–7 Mobilized as well as infused progenitor cells are selectively recruited to ischemic tissue, whereas their incorporation into nonischemic heart or hind limb tissue is an extremely rare event.8 The recruitment and incorporation of progenitor cells is augmented by growth factors and chemokines such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor 1 (SDF-1), which are both upregulated in ischemia tissue.9–13 Endothelial progenitor cells (EPCs) are well endowed with receptors for VEGF (VEGF receptor 2) and SDF-1 (CXCR4), which allows them to sense their way through the circulation to home to sites of acute ischemic injury. In contrast to acute ischemia, however, nonischemic or chronic ischemic tissue is characterized by a markedly lower expression of chemoattractant factors resulting in a reduced capacity to recruit a reasonable number of EPCs.14–17
Clinical Perspective p 2830
High-energy extracorporeal shock wave (SW) therapy is commonly used to fragmentize kidney stones for >20 years.18 At lower energy levels, however, SWs exert distinct vascular effects such as augmentation of neovascularization in a pig model of myocardial infarction.19 Moreover, a first clinical study in patients with severe coronary artery disease indicates that repetitive extracorporeal cardiac SW therapy may improve myocardial perfusion and clinical symptoms.20 These vascular effects are most likely mediated through enhanced expression of VEGF and nitric oxide.19,21–23 Because VEGF is a potent chemoattractant for progenitor cells, we reasoned that preconditioning of the target tissue with low-dose energy SW therapy may enhance EPC recruitment.
Indeed, our data demonstrate that low-energy SW therapy is capable of augmenting expression of VEGF and SDF-1 and subsequently enhancing recruitment of systemically infused progenitor cells to nonischemic and, most important, chronic ischemic tissue.
Methods
Isolation of EPCs
EPCs were isolated and cultivated as described previously.24 In short, mononuclear cells were isolated by density-gradient centrifugation from human peripheral blood with Biocoll separating solution (density 1.077; Biochrom AG, Berlin, Germany). Immediately after isolation, 8×106 mononuclear cells/mL medium were plated on fibronectin-coated culture dishes and maintained in endothelial basal medium (Cambrex, Verviers, Belgium) supplemented with endothelial growth medium SingleQuots (Cambrex) and 20% fetal calf serum (Gibco, Berlin, Germany). After 3 days in culture, nonadherent cells were removed by thorough washing with PBS. Adherent EPCs were characterized by dual-staining for 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein and lectin and by the expression of endothelial marker proteins KDR, VE-cadherin, eNOS, and von Willebrand factor.25
Animals
To inject xenogenic human EPCs into rats, we used athymic immunocompromised nude rats (Hsd:RH-Foxn1rnu; Harlan, Borchen, Germany) at the age of 5 weeks. During SW therapy, animals were anesthetized with medetomidine (0.1 mg/kg) and were administered carprofen (5 mg/kg) for analgesia.
Chronic Hind Limb Ischemia Model
The in vivo neovascularization capacity of EPCs was investigated in a rat model of unilateral hind limb ischemia. The proximal portion of the right femoral artery, including the superficial and the deep branch as well as the distal portion of the saphenous artery were occluded with an electrical coagulator. The overlying skin was closed with surgical staples. After about 3 weeks, SW preconditioning was performed. Twenty-four hours after SW therapy, a total dose of 106 EPCs was intravenously injected. Limb perfusion was assessed after 2 weeks using laser Doppler imaging (Moore, Axminster, UK).
SW Treatment
The SW device (Dornier Biotripter, Wessling, Germany) is a prototype based on a commercially available lithotripter that was equipped with a newly developed low-energy SW source. The SW source comprised an integrated ultrasound transducer for image-guided localization of the treatment area. The focal point of the SW source was indicated by a cross-hair on the ultrasound monitor adjusted to the center of the adductor muscle. The penetration depth was adjusted using an inflatable bellow under control of the integrated ultrasound. The pressure field generated by this device is characterized by an ellipsoidal cigar-shaped focal zone (“line focus”) in the plane perpendicular to the direction of SW propagation. We applied an energy flux density of 0.05 mJ/mm2 and delivered 500, 1000, and 2000 impulses. In some animals, hind limb ischemia was employed instead of SW therapy as a positive control. If subsequent cell therapy was performed, a total of 106 EPCs were intravenously injected 24 hours after SW preconditioning or induction of hind limb ischemia.
Polymerase Chain Reaction
Twenty-four hours after SW application, animals were euthanized and adductor muscles from the treated right and untreated left hind limb were harvested. Total RNA (RNA minikit, Qiagen, Hilden) was isolated from snap-frozen tissue and was subjected to polymerase chain reaction (Applied Biosystems, Foster City, Calif). To amplify rat SDF-1, we used the following primers: rat SDF-1 sense 5′-GCCCCTGCCGATTCTTTGAG -3′, antisense 5′-GTCCAGGTACT-CTTGGATCCAC-3′ (SDF-1 band 164 bp); GAPDH sense 5′-TCA-CCATCTTCCAGGAGCGAGATC-3′, antisense 5′-GAGACCACC-TGGTGCTCAGTGTAG-3′ (GAPDH band 511 bp). Polymerase chain reaction reagents were from Invitrogen (Karlsruhe, Germany). Polymerase chain reaction products were analyzed on 3% and 1.5% agarose gels for SDF-1 and GAPDH, respectively.
Histological Analysis
The harvested tissues were embedded in paraffin or cryopreserved in OCT compound (TissueTek, Sakura, The Netherlands), followed by preparation of 5-μm cryosections, fixation in acetone for 2 minutes, and blocking for 30 minutes in PBS+1% BSA. To assess the morphological integrity, paraffin-embedded tissue was analyzed by hematoxylin & eosin staining. Moreover, immunohistochemistry for the membrane marker laminin was used to examine the integrity of the cellular membrane. For quantification of apoptotic cells, we used an in situ DNA nick-end labeling assay kit from Roche (Mannheim, Germany) according to the manufacturer’s instructions.
Recruitment of infused EPCs was assessed by labeling EPCs with red fluorescent CM-Dil prior to intravenous injection and then by staining the cryosections for the endothelial marker anti–von Willebrand factor–FITC (1:50; Acris, Hiddenhausen, Germany), the membrane marker anti-laminin (1:50; Invitrogen) followed by anti-rabbit immunoglobulin Alexa647 (1:400; Invitrogen) and nuclear staining with Sytox blue (1:2000; Invitrogen).
VEGF staining was performed with anti-VEGF antibody in frozen sections (1:50 in PBS+1% BSA; R&D Systems, Minneapolis, Minn) overnight at 4°C. After washing twice with PBS, the secondary step antibody anti-mouse Alexa 488 (1:200, Invitrogen) and the nuclear stain Sytox (1:2000, Invitrogen) were incubated for 60 minutes at room temperature. Samples were analyzed with an LSM 510 confocal microscope (Zeiss, Jena, Germany).
Statistical Analysis
Results are expressed as mean±SEM. Overall comparison of the treatment groups was performed with the Kruskal-Wallis test followed by post hoc pairwise comparison using the Mann-Whitney test. Probability values <0.05 were considered statistically significant. All analyses were performed with SPSS 11.5 (SPSS Inc, Chicago, Ill).
The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript written.
Results
Effect of In Vivo SW Treatment on the Expression of Chemoattractant Factors
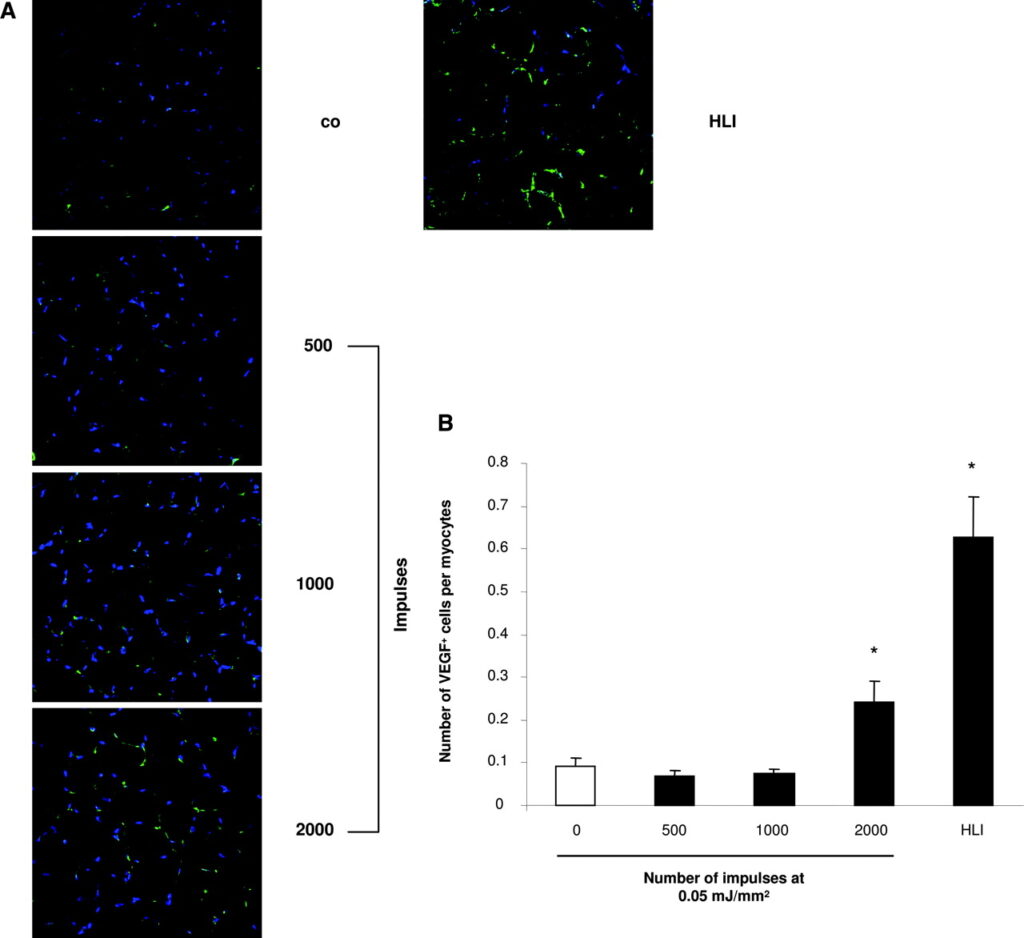
First, we investigated whether SW therapy exerts a preconditioning effect by inducing chemoattractant factors in the targeted nonischemic tissue. In nude rats, SWs at the low-energy energy flux density of 0.05 mJ/mm2 with an increasing impulse rate that ranged from 500 to 2000 were applied to the adductor muscles of right hind limbs. The untreated left hind limbs were used as negative controls, whereas induction of acute hind limb ischemia in other animals served as positive controls. In untreated hind limbs, mRNA expression of the chemoattractant factor SDF-1 was below the detection limit (Figure 1A and 1B). SW treatment significantly upregulated SDF-1 mRNA expression in nonischemic hind limbs when ≥1000 impulses were applied. Importantly, the increased level of SDF-1 mRNA expression in SW-treated hind limbs was comparable to levels observed in hind limbs subjected to severe acute ischemia by ligation of the superficial and profound femoral artery. The increase in SDF-1 expression induced by SW treatment was detected in and around von Willebrand factor–positive vessels but also in surrounding myocytes by immunostaining (data not shown). Moreover, SW treatment significantly upregulated the number of VEGF+ cells per myocyte detected on frozen sections from hind limbs treated at impulse numbers >1000 impulses (Figure 2A and 2B).

Effect of SW Treatment on Tissue Morphology and Apoptosis
To exclude tissue damage as a potential side effect of SW treatment, we analyzed the hind limb muscles by hematoxylin & eosin staining (Figure 3A) as well as by membrane staining with an antibody against laminin (Figure 3B), and determined the number of apoptotic cells (Figure 3C) and inflammatory cells (data not shown)24 hours after application of an energy flux density of 0.05 mJ/mm2 at impulse rates ranging from 500 to 2000. Neither the histologic morphology, the number of apoptotic cells, nor the invasion of inflammatory cells was affected by SW treatment, excluding a major tissue injury induced by low-dose SW therapy.

Effect of SW-Mediated Preconditioning on EPC Recruitment
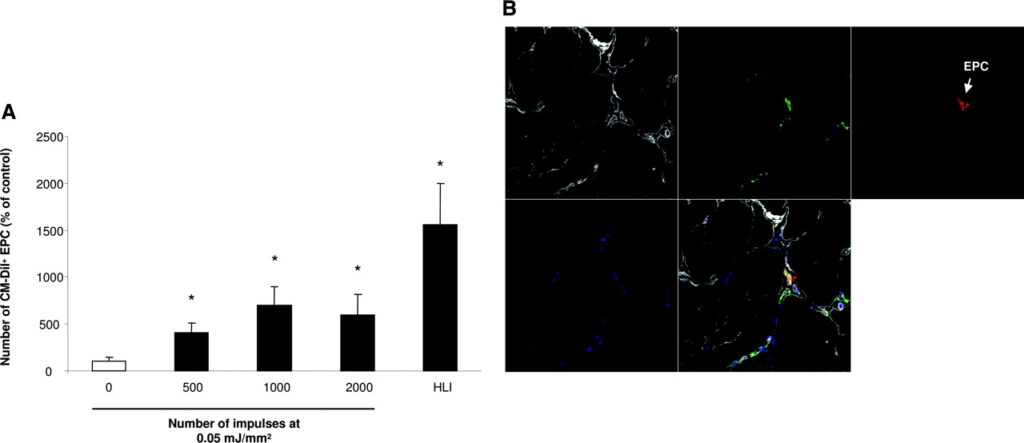
To test the hypothesis that the SW-induced upregulation of chemoattractants in the adductor muscles is functionally relevant for enhancement of the recruitment of intravenously injected circulating EPCs, we pretreated the nonischemic muscles of the right hind limb with SWs (0.05 mJ/mm2 at an impulse rate between 500 and 2000) followed by intravenous infusion of 106 human CM-Dil–labeled EPCs after 24 hours. Animals were euthanized 24 hours after administration of the cells. Confocal analysis of the histologic sections revealed a significantly increased number of red fluorescent EPCs in the preconditioned adductor muscles (Figure 4A and 4B).
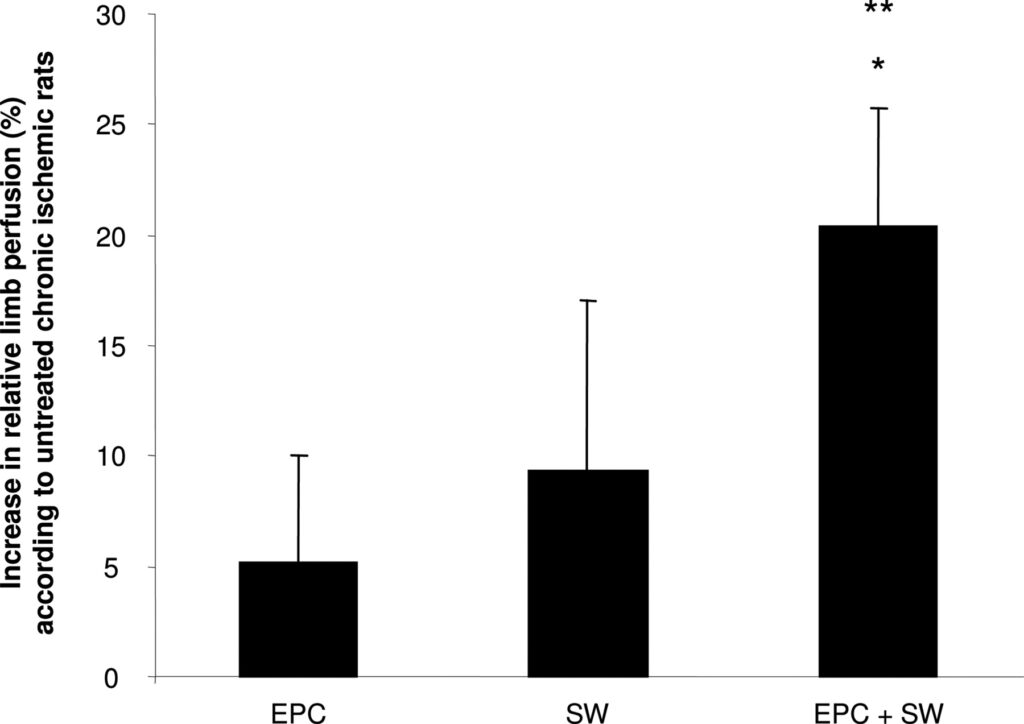
To further investigate enhanced EPC homing in a more clinically relevant setting, we used a model of unilateral chronic hind limb ischemia in nude rats. In this model, the recovery of blood flow after induction of limb ischemia reached a plateau after 3 weeks with a relative limb perfusion of 70% (ratio ischemic/nonischemic limb). Thus, we initiated our experiments to investigate the effects of SW-facilitated cell therapy at least 3 weeks after induction of hind limb ischemia. Twenty-four hours after preconditioning of the chronic ischemic adductor muscles by SW treatment, 106 human EPCs were intravenously injected. After 14 days, we assessed changes in limb perfusion by laser Doppler imaging. Neither EPC injections alone (increase relative to the control group: 5.1±4.9%, P=0.6) nor SW treatment alone (9.3±7.7%, P=0.3) significantly enhanced perfusion of chronic ischemic limbs compared with nontreated rats. In contrast, SW preconditioning 24 hours before EPC injection resulted in a significantly enhanced blood flow recovery in the chronic ischemic hind limb as compared with untreated limbs (Figure 5).
Discussion
The present study demonstrates that pretreatment of the target tissue with low-energy SW therapy facilitates recruitment of infused progenitor cells into nonischemic tissue and, most important, into chronic ischemic tissue. Functionally, in a rat model of chronic hind limb ischemia, which mimics the clinical setting of patients with chronic ischemia, the combined treatment with low-energy SWs followed by intravenously administered EPCs translated into enhanced blood flow recovery. These data demonstrate proof-of-principle for the use of SW preconditioning for improving the efficacy of cell therapy to enhance neovascularization, and thus could have major implications for the emerging field of regenerative medicine.
Low-energy SW therapy alone effectively induced angiogenesis and ameliorated chronic ischemia in animals and humans without cell therapy,19,20 possibly by augmenting the expression of proangiogenic cytokines such as VEGF. Mechanistically, we not only confirm these previous studies with respect to upregulation of VEGF19 but also demonstrate that low-energy SWs significantly upregulate expression of the chemoattractant SDF-1 in treated muscles. SDF-1 is a specific ligand for CXCR4, which is strongly expressed on EPCs and hematopoietic cells and plays a crucial role in cell homing and function.26–29 Specifically, EPCs derived from mice with a heterozygous expression of CXCR4 (homozygous knockout mice are lethal) exhibit a significantly lower vascular incorporation rate and fail to augment neovascularization after ischemia.27 Moreover, overexpression of the CXCR4 ligand SDF-1 plays a key role for trapping and positioning a subpopulation of circulating proangiogenic myeloid cells around the growing vessels in tissues.12 In this model, VEGF is critically involved in the recruitment of a heterogeneous mix of myeloid cells from the bone marrow to the blood as VEGF overexpression also induces expression of SDF-1 in mural cells around blood vessels. Then, a subpopulation of bone marrow–derived cells that express CXCR4 is recruited, captured, and positioned around the vessels that express SDF-1. This proangiogenic subpopulation stimulates endothelial cell sprouting by release of matrix metalloproteinase-9 and other angiogenic factors.
In our experiments, histological analysis revealed that homing of circulating EPC to untreated nonischemic tissue was an extremely rare event. We already obtained a significant increase in the number of retrieved EPCs in tissues treated with 500 impulses; however, EPC recruitment usually follows the enhanced expression of SDF-1, which was observed at 1000 impulses, whereas a significant increase in VEGF expression was only observed for the application of 2000 impulses. Together with the fact that EPCs strongly express the CXCR4 receptor,27 these data suggest that the SW-induced SDF-1 expression might be the predominant mechanism for the enhanced recruitment of EPCs after SW treatment.
Intriguingly, in a rat model of chronic hind limb ischemia, the neovascularization-enhancing effect of EPC therapy, which was virtually lost in chronic ischemic tissue, was rescued by pretreatment of the tissue with low-energy SW therapy. Systemic infusion of EPCs alone did not result in a significant improvement in the recovery of the blood flow in the chronic ischemic limb. The lack of efficacy of EPC infusion alone is not much of a surprise as, in this model at the time of EPC treatment, the enhanced expression of chemoattractants after induction of ischemia has already returned to baseline levels.17 This can be well rationalized by the observation that EPC mobilization peaks on day 7 after onset of acute myocardial infarction and returns to baseline levels thereafter.30 Therefore, in our setting of chronic limb ischemia, EPC recruitment after systemic infusion was most likely insufficient to result in a detectable enhancement of limb perfusion recovery. Because most patients suitable for cell therapy do not suffer from acute tissue ischemia but rather endure chronic tissue ischemia, our study design certainly bears great clinical relevance. Indeed, recent studies demonstrate that the cardiac improvement induced by infused EPC in patients with chronic myocardial infarction is markedly reduced as compared with patients with acute myocardial infarction.1,5,31 In the present study, we were able to demonstrate consistently that the combination of low-energy SW treatment and cell therapy in chronic ischemic tissue resulted in a significant stimulation of blood flow recovery in chronic ischemic limbs, whereas EPC treatment alone did not.
Previous work by Hiasa et al32 has already taken advantage of the cell recruitment–enhancing properties of SDF-1 by transfecting plasmid DNA encoding for SDF-1 into ischemic muscles. Their studies demonstrated that SDF-1 gene transfer augments ischemia-induced vasculogenesis and angiogenesis in vivo. The delivery of vectors to allow for targeted expression of growth factors or cytokines has been challenging, particularly in the clinical setting. In contrast, SW therapy is a noninvasive technique, widely used for clinical treatment without major complications, and does not require introduction of genetic material.33 High-energy extracorporeal SW therapy, as opposed to the low-energy modus used for the present study, has been commonly used to fragmentize kidney stones for >20 years.18 SWs are amplitudes of acoustic pulse waves similar to ultrasound with extremely high positive pressures (up to 100 MPa) and negative pressure of about 10 MPa. Cavitation bubbles are formed in the trailing, negative pressure cycle after the SW front. When the cavitation bubble collapses, a transient SW emission and jet formation is generated.34 Beside cavitation, shear forces are generated through the transient pressure pulse. Whereas cavitation is known to increase permeability of the cell membrane, shear forces could result in induction of gene expression. However, this issue is still a matter of debate and certainly deserves further investigation. With respect to low-energy SWs, which share the physical features of high-energy SWs, initial clinical pilot data indicate that repetitive extracorporeal cardiac SW therapy in patients with severe coronary artery disease is safe and feasible.20 In the present study, low-energy SWs consistently did not induce tissue damage or apoptosis, which provides further evidence that low-energy SW treatment is generally well tolerated. Therefore, one may speculate that preexposure of hearts or limbs to low-energy SWs prior to intravascular cell therapy may be well suited to augment stem and progenitor cell recruitment to chronic or even nonischemic tissue (eg, dilative cardiomyopathy), and to subsequently enhance functional recovery in these patients.
We thank Marion Muhly-Reinholz, Ariane Fischer, and Tino Röxe for their excellent technical assistance. We are grateful to Dr Andreas Lutz (Dornier MedTech Systems GmbH) for helpful discussions.
Sources of Funding
This work was supported by the Deutsche Forschungsgemeinschaft (FOR 501: He3044/2-2, Wa1461/2-2, and TR23), the Alfried Krupp-Stiftung, and the Leducq Foundation.
Disclosures
Drs Aicher, Heeschen, Dimmeler, and Zeiher are named as co-inventors on a patent application (Shock wave pretreatment as a therapeutic tool for targeted recruitment of stem/progenitor cells) filed by Dornier MedTech Systems GmbH.
Footnotes
Correspondence to Stefanie Dimmeler, PhD, Department of Medicine III, University of Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany. E-mail dimmeler@em.uni-frankfurt.de
References
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002; 106: 3009–3017.LinkGoogle Scholar
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002; 106: 1913–1918.LinkGoogle Scholar
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364: 141–148.CrossrefMedlineGoogle Scholar
- Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005; 46: 1651–1658.CrossrefMedlineGoogle Scholar
- Assmus B HJ, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin M, Abolmaali N, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006; 355: 1222–1232.CrossrefMedlineGoogle Scholar
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006; 367: 113–121.CrossrefMedlineGoogle Scholar
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003; 361: 47–49.CrossrefMedlineGoogle Scholar
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275: 964–967.CrossrefMedlineGoogle Scholar
- Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000; 342: 626–633.CrossrefMedlineGoogle Scholar
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003; 107: 1322–1328.LinkGoogle Scholar
- De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004; 104: 3472–3482.CrossrefMedlineGoogle Scholar
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006; 124: 175–189.CrossrefMedlineGoogle Scholar
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999; 18: 3964–3972.CrossrefMedlineGoogle Scholar
- Ruck A, Gustafsson T, Norrbom J, Nowak J, Kallner G, Soderberg M, Sylven C, Drvota V. ANP and BNP but not VEGF are regionally overexpressed in ischemic human myocardium. Biochem Biophys Res Commun. 2004; 322: 287–291.CrossrefMedlineGoogle Scholar
- Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, Christensen G, Froland SS, Attramadal H, Gullestad L, Aukrust P. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000; 47: 778–787.CrossrefMedlineGoogle Scholar
- Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, Holm AM, Halvorsen B, Froland SS, Gullestad L, Aukrust P. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002; 106: 36–42.LinkGoogle Scholar
- Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, Wang K, Zou Y. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005; 100: 217–223.CrossrefMedlineGoogle Scholar
- Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982; 127: 417–420.CrossrefMedlineGoogle Scholar
- Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, Matsumoto Y, Kajihara N, Eto M, Matsuda T, Yasui H, Takeshita A, Sunagawa K. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004; 110: 3055–3061.LinkGoogle Scholar
- Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, Takeshita A, Sunagawa K, Shimokawa H. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis. 2006; 17: 63–70.CrossrefMedlineGoogle Scholar
- Gotte G, Amelio E, Russo S, Marlinghaus E, Musci G, Suzuki H. Short-time non-enzymatic nitric oxide synthesis from L-arginine and hydrogen peroxide induced by shock waves treatment. FEBS Lett. 2002; 520: 153–155.CrossrefMedlineGoogle Scholar
- Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, Yang LC. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003; 21: 984–989.CrossrefMedlineGoogle Scholar
- Park JK, Cui Y, Kim MK, Kim YG, Kim SH, Kim SZ, Cho KW. Effects of extracorporeal shock wave lithotripsy on plasma levels of nitric oxide and cyclic nucleotides in human subjects. J Urol. 2002; 168: 38–42.CrossrefMedlineGoogle Scholar
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001; 108: 391–397.CrossrefMedlineGoogle Scholar
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003; 108: 2511–2516.LinkGoogle Scholar
- Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000; 105: 101–111.CrossrefMedlineGoogle Scholar
- Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005; 97: 1142–1151.LinkGoogle Scholar
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004; 10: 858–864.CrossrefMedlineGoogle Scholar
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003; 362: 697–703.CrossrefMedlineGoogle Scholar
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001; 103: 2776–2779.CrossrefMedlineGoogle Scholar
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004; 44: 1690–1699.CrossrefMedlineGoogle Scholar
- Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004; 109: 2454–2461.LinkGoogle Scholar
- Skolarikos A, Alivizatos G, de la Rosette J. Extracorporeal shock wave lithotripsy 25 years later: complications and their prevention. Eur Urol. 2006; 50: 981–990.CrossrefMedlineGoogle Scholar
- Ogden JA, Toth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001: 8–17.MedlineGoogle Scholar
circulationahaCirculationCirculationCirculation0009-73221524-4539Lippincott Williams & WilkinsCLINICAL PERSPECTIVE19122006
Stem and progenitor cell therapy is a novel approach to improve neovascularization and function of ischemic tissue. Enhanced tissue expression of chemoattractant factors such as stromal cell–derived factor 1 and vascular endothelial growth factor is crucial for the recruitment of circulating endothelial progenitor cells (EPCs) during acute ischemia. In chronic ischemia, however, expression of these chemoattractants is less pronounced, which results in insufficient EPC recruitment into the target tissue. Therefore, we investigated the effect of targeted extracorporeal shock wave application to facilitate EPC recruitment into nonischemic and chronic ischemic tissue. Preconditioning of both nonischemic and chronic ischemic tissue with low-energy shock waves improves recruitment of circulating EPCs via enhanced expression of chemoattractant factors such as vascular endothelial growth factor or stromal cell–derived factor 1. Functionally, in a rat model of chronic hind limb ischemia, which mimics the clinical setting of patients with chronic ischemia, the combined treatment with low-energy shock waves followed by intravenously administered EPCs translated into enhanced blood flow recovery. These data demonstrate proof-of-principle for the use of shock wave preconditioning to improve the efficacy of cell therapy to induce neovascularization, and, thus, could have major implications for the emerging field of regenerative medicine.
*The first 2 authors contributed equally to this work.
Alexandra Aicher, Christopher Heeschen, Ken-ichiro Sasaki, Carmen Urbich, Andreas M. Zeiher and Stefanie Dimmeler Circulation 2006;114;2823-2830; originally published online Dec 4, 2006; DOI: 10.1161/CIRCULATIONAHA.106.628623 Circulation is published by the American Heart Association. 7272 Greenville Avenue, Dallas, TX 72514 Copyright © 2006 American Heart Association. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539
