Accelerated Healing of 2nd-Degree Burns with Shockwave Therapy
Authors: Christian Ottomann, MD,∗ Alexander Stojadinovic, MD, FACS, Philip T. Lavin, PhD, Francis H. Gannon, MD, Michael H. Heggeness, MD, Richard Thiele, MD, Wolfgang Schaden, MD, and Bernd Hartmann, MD
Summary: This study investigated the effects of extracorporeal shock wave therapy (ESWT) on burn wounds to determine if it could enhance the healing process. A group of 50 patients with acute second-degree burns was randomly divided into two groups: one receiving standard therapy (wound cleaning and antiseptic treatment) alone, and the other receiving standard therapy along with defocused ESWT. The time taken for the burn wounds to completely heal was measured as the primary endpoint.
The results showed that patients who underwent ESWT achieved complete healing (epithelialization) of their burn wounds in an average of 9.6 days, compared to 12.5 days for those who did not receive ESWT. This significant reduction in healing time suggests that shockwave therapy can accelerate the process of wound closure. Even after accounting for differences in age between the two groups, the accelerated healing effect of ESWT remained statistically significant.
The study concluded that a single session of defocused shockwave treatment, administered after wound cleaning and antiseptic therapy, can significantly speed up the healing of superficial second-degree burn wounds. However, further research is needed to confirm these findings in a larger study (phase III trial). The promising results of this study indicate that shockwave therapy could be a valuable addition to the treatment of burn wounds, potentially improving outcomes for patients.
Read more below:
Background: As extracorporeal shock wave therapy (ESWT) can enhance healing of skin graft donor sites, this study focused on shock wave effects in burn wounds.
Methods: A predefined cohort of 50 patients (6 with incomplete data or lost to follow-up) with acute second-degree burns from a larger study of 100 patients were randomly assigned between December 2006 and December 2007 to receive standard therapy (burn wound debridement/topical antiseptic therapy) with (n = 22) or without (n = 22) defocused ESWT (100 impulses/cm2 at 0.1 mJ/mm2) applied once to the study burn, after debridement. Randomization sequence was computer-generated, and patients were blinded to treatment allocation. The primary endpoint, time to complete burn wound epithelialization, was determined by independent, blinded-observer. A worst case scenario was applied to the missing cases to rule out the impact of withdrawal bias.
Results:
Patient characteristics across the 2 study groups were balanced (P > 0.05) except for older age (53 ± 17 vs. 38 ± 13 years, P = 0.002) in the ESWT group. Mean time to complete (≥95%) epithelialization (CE) for patients that did and did not undergo ESWT was 9.6 ± 1.7 and 12.5 ± 2.2 days, respectively (P < 0.0005). When age (continuous variable) and treatment group (binary) were examined in a linear regression model to control the baseline age imbalance, time to CE, age was not significant (P = 0.33) and treatment group retained significance (P < 0.0005). Statistical significance (P = 0.001) was retained when ESWT cases with missing follow-up were assigned the longest time to CE and when controls with missing follow-up were assigned the shortest time to CE.
Conclusions:
In this randomized phase II study, application of a single defocused shock wave treatment to the superficial second-degree burn wound after debridement/topical antiseptic therapy significantly accelerated epithelialization. This finding warrants confirmation in a larger phase III trial (ClinicalTrials.gov identifier: NCT01242423).
(Ann Surg 2012;255:23–29)
Advances in the treatment of the thermally injured patient including timing and extent of fluid resuscitation, early burn wound excision and grafting, topical antibiotic and biosynthetic therapy, application of skin substitutes, and critical care in burn centers of
excellence have impacted significantly the clinical outcomes of these patients. Nature and intensity of treatment is directed according to burn wound location, extent, and depth. Most superficial-thickness
burns heal within 2 weeks of injury and are managed effectively with debridement, topical antiseptic therapy, and biologic or nonbiologic dressings. These measures reduce contamination, decrease insensible fluid losses, improve pain, and accelerate reepithelialization. Many research efforts have focused on accelerating partial-thickness burn wound healing through the use of growth factors and other topical treatment modalities.1 ,2
The noninvasive modality, extracorporeal shock wave therapy
(ESWT), may improve perfusion and accelerate epithelialization in burn wounds; however, few studies have addressed the clinical utility of this approach.3,4
We have recently shown in an exploratory
phase II randomized trial that defocused ESWT applied immediately after split-thickness harvest of skin graft accelerates graft donor site epithelialization.5
The current phase II randomized trial was conducted to determine if similar accelerated reepithelialization could
be attained on burn wounds through the single application of shockwaves after superficial-thickness burn wound debridement.
METHODS
This report complies with the reporting standards established by the revised Consolidated Standards of Reporting Trials
(CONSORT) consensus statement.6
Participants
A prospective randomized phase II clinical trial was conducted
from December 2006 to December 2007, which was approved by
the Charite Berlin Ethics Committee, under authorization number ´
EA/160/06. During the study period, 100 patients were enrolled, 50
patients with donor sites treated with standard of practice with or
without ESWT, and 50 patients with superficial second-degree burns
to this study who provided informed consent. There were no refusals
to enroll in the study among those approached for study participation. Once eligibility was confirmed, study patients were assigned
randomly to 1 of 2 study groups. The control group underwent burn
wound debridement of devitalized skin (epidermis) and ruptured blisters and daily antiseptic dressing changes involving application of
topical nonadherent silicone mesh (Mepitel) and antiseptic gel (Polyhexanide/Octenidine) until complete epithelialization, according to institutional standards of practice. The shock wave group underwent the same treatment in addition to a single application of unfocused shock wave therapy to the study burn. Eligible patients were
nonpregnant women or men, between 18 and 80 years of age, capable
of providing informed consent. Eligible patients were also those with
second-degree burns (superficial second degree: involving epidermis
and extending into dermis). Determination of burn wound depth was
established on clinical grounds on the basis of the color and burn
wound appearance, capillary refill, and sensation. Superficial seconddegree burns appear erythematous, possibly blistered, have capillary
refill, and are sensate to pin prick testing. Laser doppler imaging was
not used in this study to determine burn wound depth. First, second
degree deep dermal, and third-degree burn wounds were excluded
from study as were patients with insulin-requiring diabetes mellitus, dialysis dependent renal failure, ongoing systemic therapy for
malignancy, systemic dermatologic disease, ongoing corticosteroid
therapy, and active drug abuse. Six of the 50 study patients enrolled
were excluded from final analysis because of incomplete data or loss
to follow-up. There are 44 evaluable patients, who were blinded to
treatment allocation, and analyzed on an intent-to-treat basis.
Shock Wave Administration
After study burn wound debridement, shock wave therapy was
administered as a single treatment within 24 hours of superficial
second-degree burn wound debridement to patients randomized to
the ESWT intervention arm of the study. The shock waves were
delivered to the superficial second-degree burn wound as a single
treatment. The administered shock wave dose was 100 impulses/cm2
(according to burn wound surface area) using an energy flux density
of 0.1 mJ/mm2, administered at 20 seconds/cm2. Sterile ultrasound
conducting gel (Lavasept gel) was applied to the burn wound surface.
A sterile plastic protective film was placed over the wound. Ultrasound
gel was then applied onto the drape as a coupling media. Unfocused
shock waves were applied through the conducting gel and sterile film
directly to the debrided superficial second-degree burn wound, using
the OW180C DermaGold [MTS Europe GmbH, Tissue Regeneration
Technologies, LLC, Woodstock, GA, which is a certified medical
device in Europe (TUV Rheinland CE 1275)].
Primary Outcome (Burn Wound Epithelialization)
Assessment
Study participants that have provided informed consent to participate in this clinical trial were followed in-hospital daily until discharge and were evaluated 12 weeks after hospital discharge in outpatient clinic. Complete burn wound healing was defined as more
than or equal to 95% reepithelialization. Study patients were monitored carefully during the follow-up period for cardiac, neurological,
dermal, thermal, or allergic reactions or adverse events.
Objective
The principal aim of this study was to determine if a single
application of defocused ESWT to a superficial second-degree burn
wound can accelerate reepithelialization over our current standard
of practice. The prospective hypothesis tested (H0) was: there is no
difference in time to complete reepithelialization between ESWT
and control; versus (H1), there is a reduction in time to complete
reepithelialization for ESWT versus control.
Outcomes
The primary outcome variable was time to complete burn
wound healing (≥95% reepithelialization).
Sample Size
The sample size was prospectively set at 50 patients to detect
a reduction in mean time to ≥95% reepithelialization consistent with
a mean 2-day reduction for ESWT and a 2 to 2.5 day standard deviation (SD) for the time to ≥95% reepithelialization; no formal power
calculation was originally performed. On the basis of the actual study
data, the current study had more than 80% power to detect a 0.85
effect size for the treatment differences in mean time to healing of 2
days between the 2 study arms using a 2-sided test with 5% type I
error.
Randomization and Determination of Primary Study
Endpoint
Patients were randomized in a 1:1 ratio using Rancode 3.6
Professional (IDV, Gauting, Germany) to undergo superficial seconddegree burn wound therapy in accordance with institutional standards specified above or the same treatment with a single, unfocused shock wave treatment at the aforesaid parameters. All patients
were treated as randomized. Randomization was achieved through a
computerized randomization system (without stratification) based on
random number generation. The randomization sequence was concealed until study group assignment. Study participants were blinded
to treatment assignment. The primary endpoint, time to complete
(≥95%) wound reepithelialization, was determined by an independent, blinded-observer. This observer is a highly trained professional,
senior plastic surgeon, with experience in complex burn and wound
care. Serial digital images of study wounds were reviewed by an
expert in wound care, blinded to treatment group assignment, who
determined completeness of study wound epithelialization. Both independent reviewers were given the photos of the study wounds to
assess the time to complete reepithelialization. The interclass correlation was 0.986 for the time to ≥95% reepithelialization.
Statistical Methods
Summary statistics were obtained using established methods.
Categorical variables between groups were compared using a 2-sided
Fisher exact test. Continuous baseline data were presented as means
and standard deviations (mean ± SD) with medians and ranges for
each treatment group and compared using an unpaired t test or a
Wilcoxon rank sum test. The primary outcome variable in this study
was time to superficial second-degree burn wound reepithelialization, which was defined as time from initial debridement/application
of ESWT to the first documentation of complete study superficial
second-degree burn wound healing (≥95% reepithelialization). Mean
time to burn wound epithelialization ( ± SD) was compared between
study groups according to an unpaired t test; analysis of covariance
was performed using age (continuous variable) and treatment group
(binary) together as independent predictors of the dependent variable,
time to complete epithelialization, to control for the age imbalance in
comparing treatment groups. Statistical analysis was performed using JMP(v8) and SAS software (JMP and SAS, Cary, NC). A 2-sided
P ≤ 0.05 was considered significant.
RESULTS
Patients
Between December 2006 and December 2007, 50 study patients were enrolled and provided informed consent. No patient approached for study participation refused to enroll in the study. Study
patients were randomly assigned to undergo standard institutional
treatment of the superficial second-degree burn wound consisting of
debridement of devitalized skin (epidermis) and ruptured blisters and
daily antiseptic dressing changes or the same treatment in addition
to a single application of unfocused ESWT (100 impulses/cm2) to the study second-degree burn. All patients were treated as randomized. All patients of the ESWT group were treated within 24 hours of
admission to the burn unit.
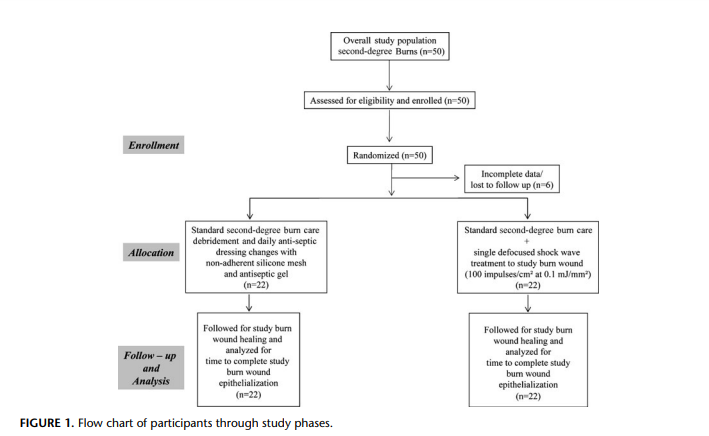
Not all study patients were available for final analysis. Six of
the 50 study patients were excluded from final analysis because of
incomplete data (1 patient) or loss to follow up (5 patients), leaving
44 evaluable patients, who were analyzed on an intent-to-treat basis
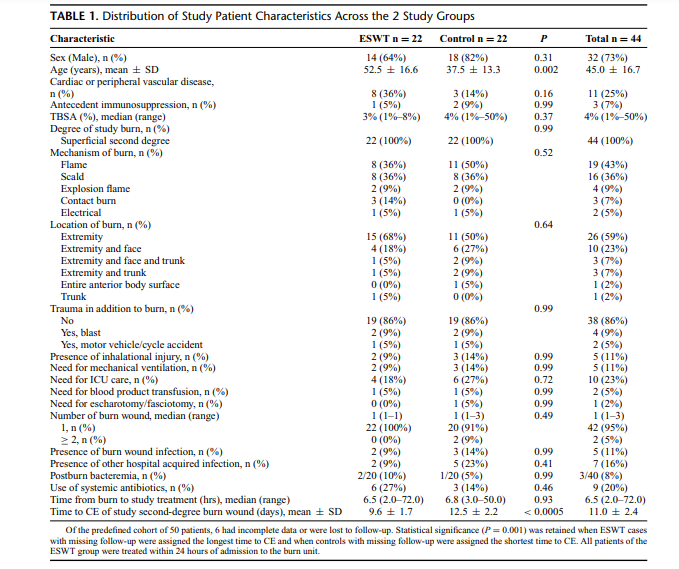
(Fig. 1). Baseline demographic characteristics of the 44 evaluable
study patients are shown in Table 1. Patients were predominantly men
with median total body surface area (TBSA) involved by thermal injury of 3% to 4%. Involved anatomic location by burn was mostly
the extremity and face, and the majority (95%) of patients underwent only a single burn wound procedure; when 2 patients underwent
a second wound procedure, the worst wound was used as the index
wound for the primary efficacy analysis. Patient characteristics across
the 2 study groups were balanced (P > 0.05) except for older age
(53 ± 17 years vs. 38 ± 13 years, P = 0.002) in the ESWT
group.
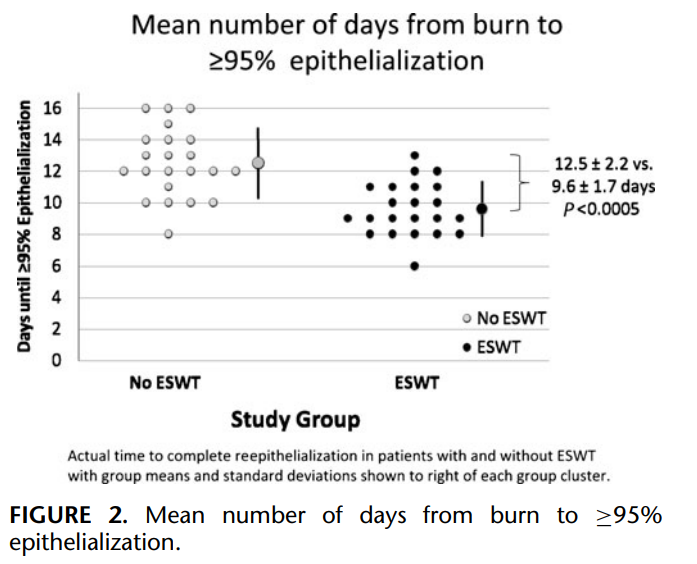
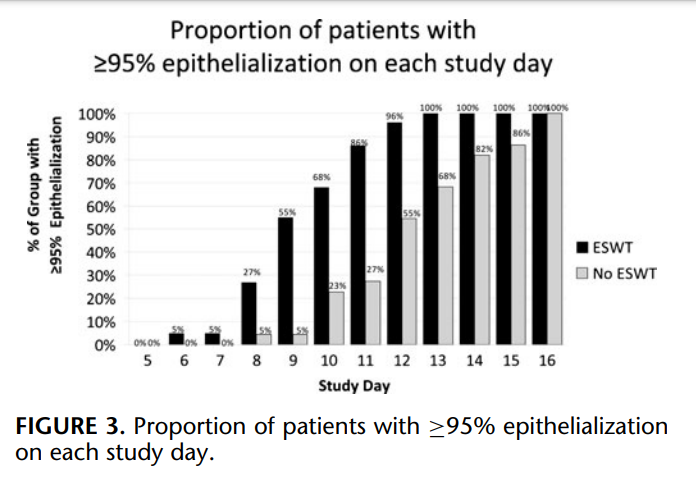
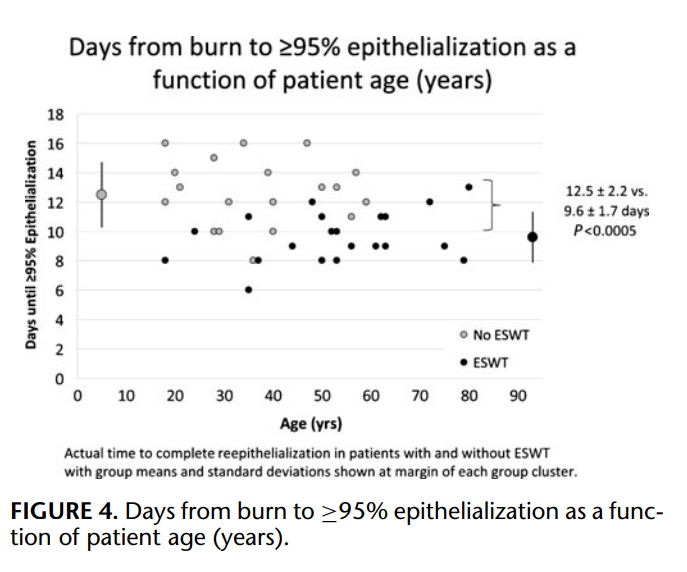
Primary Outcome Assessment
All superficial second-degree burn wounds healed over a mean period of 11.0 ± 2.4 days. Mean time to complete second-degree burn wound epithelialization for patients that did and did not undergo ESWT was 9.6 ± 1.7 and 12.5 ± 2.2 days, respectively (P < 0.0005; Fig. 2). The proportion of patients with epithelialization on each study day is shown in Figure 3; 100% of shock wave treated patients healed completely by day 13, whereas 68% of patients in the control group demonstrated 100% epithelialization of the study wound. Figure 4 shows time to complete second-degree burn wound epithelialization as a function of study patient age and suggests benefit of ESWT relative to controls in the older age groups. When age (continuous variable) and treatment group (binary) were examined in a linear regression model together as independent predictors of the dependent variable, time to complete (≥95%) epithelialization, age was not significant (P = 0.33) and treatment group retained significance (P < 0.0005). When all 50 enrolled study patients are analyzed by imputing the missing data and by assuming the worst case scenario for the patients with incomplete data in terms of time to healing for each study group the mean difference in time to complete (≥95%) seconddegree burn wound epithelialization is 2.0 days [95% confidence interval (CI), 0.7–3.2], and remains significantly more rapid in the intervention arm of the study (ESWT: 10.0 ± 1.9 vs. control: 12.0 ± 2.5 days; P = 0.003); the healing time was set to 13 days for those ESWT patients with missing data and was set to 8 days for the control group subjects.
DISCUSSION
The current randomized phase II clinical trial was conducted
to determine if a single application of low-energy defocused shock
wave therapy within 24 hours of superficial second-degree-degree
burn and after debridement/topical antiseptic therapy can significantly accelerate burn wound epithelialization compared to our current standard of practice. ESWT in this study was associated with
significantly reduced time to complete (≥95%) superficial second degree burn wound healing.5
Patients receiving shock wave therapy showed significantly reduced mean time to complete (≥95%) seconddegree burn wound epithelialization (9.6 ± 1.7 vs. 12.5 ± 2.2 days; P < 0.0005) compared to the control group without ESWT. These results are similar to our recent finding of enhanced healing of skin graft donor sites treated with ESWT in a separate randomized phase II clinical trial. The current trial adds to the mounting clinical evidence supporting the hypothesis that a biomechanical stimulus can exert clinically relevant, favorable outcomes in terms of tissue repair and regeneration. Cellular mechanotransduction has been demonstrated in vitro and in animal models.7 , 8 One modality presently in clinical use that applies this principle of mechanotransduction is negative pressure wound therapy, which when applied to a wound bed, has been associated with wound bed neovascularization, increased granulation, and epithelial cell proliferation, indicative of accelerated tissue regeneration.9 , 10 Further progress pursuant to accelerated tissue repair and regeneration through mechanotransduction has been made through the application of noninvasive treatment modalities such as ultrasound and ESWT.5 , 11 –13 Experimental studies by our group and others of ESWT in wounds demonstrated shock wave-mediated proangiogenic and antiinflammatory effects in both ischemic tissues and acute burns.14 –18 One of the earliest reported studies demonstrating the positive effects of ESWT on wound healing was by Haupt et al in 1990, which showed a significant reduction in the time for reepithelialization when low energy shock waves were applied to partial thickness wounds in a porcine model, which coincided with significantly increased vascularisation of the upper dermis and thicker layer of the newly formed epithelial cells covering the wound.19 These promising findings were replicated in subsequent animal model experiments of tissue ischemia.
The positive effect of shock wave therapy on ischemic skin
flap survival was demonstrated in a rat model of ischemic epigastric skin flaps. ESWT was shown to significantly improve epigastric
skin flap survival through reduction of areas of necrotic zones; these
findings were associated with enhanced growth factor expression.20
Recent work by Takahiro et al extended these findings to improved
functional recovery in shock wave treated ischemic tissue.21 Takahiro
et al studied the effect of ESWT on ischemia-induced myocardial
dysfunction in a porcine model. The authors report that ESWT of
the ischemic myocardium was associated with complete recovery of
left ventricular ejection fraction and regional myocardial blood flow
restoration to the ischemic region within 4 weeks of ESWT.21 These
results were confirmed in humans by Fukumoto et al who successfully applied ESWT to humans with severe coronary artery disease,
which was associated with reduced myocardial ischemia.22 The suggested beneficial clinical effects of ESWT seem to extend beyond
neovascularisation, modulation of inflammation, and functional ischemic tissue recovery. The findings reported by Gerdesmeyer et al
point to a bactericidal effect of ESWT.23
Encouraged by these findings we had previously conducted a
phase II trial to assess the feasibility and safety of ESWT for acute
and chronic soft-tissue wounds. In a population of 208 patients with
complicated wounds we identified complete healing in 156 (75%)
patients undergoing a treatment protocol consisting of wound bed
preparation (debridement), outpatient, low-energy, defocused shock
wave therapy (100–1000 shocks at 0.1 mJ/mm2, according to wound
size, every 1–2 weeks over mean 3 treatments), and moist dressings. No treatment-related toxicity or infection became evident during the course of study, and no treated wound deteriorated with shock
wave therapy.13 Mean time to complete healing (100% epithelialization) varied between groups according to type of wound treated but was most rapid in burns and those with disturbed postoperative
wound healing. We concluded after this study that further testing of a
wound treatment strategy incorporating low-energy defocused shock
waves was safe and feasible, particularly in acute traumatic burns and
wounds.
We then conducted a phase II trial in patients undergoing skin
grafting. Patients were randomized to receive standard topical therapy [nonadherent silicone mesh (Mepitel) and antiseptic gel (Polyhexanide/Octenidine)] to skin graft donor sites with or without lowenergy defocused ESWT (100 impulses/cm2 at 0.1 mJ/mm2) applied
once to the donor site, immediately after skin harvest.5
Mean time
to complete graft donor site epithelialization for patients undergoing
ESWT was significantly reduced compared to controls (13.9 ± 2.0
vs. 16.7 ± 2.0 days; P = 0.0001). A single application of low-energy
defocused shock wave therapy to the graft donor site immediately after skin graft harvest may be clinically useful as it can significantly
accelerate donor site epithelialization. Future studies will assess the
impact on donor site pain, patient symptom distress, and quality of
life.
The current phase II randomized trial was conducted to determine if similar accelerated reepithelialization could be attained
through the single application of shock waves after superficial seconddegree burn wound debridement. This is the largest clinical trial published to date in patients with superficial second-degree burns treated
with ESWT. Arno et al reported their initial experience with shock
wave therapy in 15 patients with deep partial/full thickness burns.23
In that study 2 shock wave therapy sessions were applied to the deep
partial/full thickness burns on the third and fifth day after injury by using low-energy defocused ESWT (500 impulses at 0.15 mJ/mm2).23
Of all treated burns, 80% healed uneventfully before 3 weeks; as
many as 15% required surgical debridement and grafting. In our
study, 100% of shock wave treated patients with less severe superficial second-degree burns healed within 2 weeks after a single shock
wave application. Importantly Arno et al noted significantly enhanced
burn wound perfusion with laser doppler imaging after the first shock
wave treatment, findings concordant with prior mechanistic studies
demonstraing a proangiogenic effect of low-energy defocused shock
waves.
Because ESWT as a treatment for acute and chronic soft tissue wounds is in its early clinical investigational stages, the precise
mechanism of action has yet to be precisely defined. A number of
hypotheses have been proposed as to the mechanism in experimental
studies. Fukumoto et al suggests that cellular permeability changes
account partly for the positive effects of shock waves in a model of
cardiac dysfunction induced by ischemia.22 This hypothesis is also
supported by Takahiro et al, whose work group studied the effects
of ESWT on pathologically altered coronary arteries.21 Gotte et al
showed that low energy extracorporeal shock waves stimulate a rapid
increase in neuronal nitric oxide synthase (nNOS) activity and basal
nitric oxide (NO) production in a rat glioma cell line C6.24 In addition,
the treatment of C6 cells with ESWT blocks the decrease of nNOS activity and NO production induced by a mixture of lipopolysaccharides
(LPS), interferon-γ (IFN-γ ) plus tumour necrosis factor-α (TNF-α).
Shock wave treatments also downregulate nuclear factor kappa-lightchain-enhancer of activated B cells (NF-κB) activation and NF-κBdependent gene expression, including inducible NOS and TNF-α in
this C6 cell line model.24 ,
25 These findings point to a putative molecular mechanism of antiinflammatory action and a role of substance
P. Another hypothesis regarding the mechanism of actions suggested
by Mariotto et al, assumes a direct NO-triggered effect, without consecutive release of neurotransmitters, based on findings in human
umbilical vein endothelial cells of shock wave stimulated tyrosinedephosphorylation of endothelial nitric oxide synthase, ensuing
increase in NO production, and downregulation of NF-κB
activation.26
Wang et al showed that shock waves could enhance growth of
rat femur derived bone-marrow osteoprogenitor cells through tumor
growth factor beta 1 (TGF-β1) induction, and further demonstrated
the potential of shock waves to stimulate differentiation of mesenchymal progenitor cells in human umbilical cord blood into osteogenic
cell lineage through superoxide-mediated TGF-β1 production.27 ,
28
This same group of investigators demonstrated a systemic effect on
circulating growth factors in a patient population undergoing ESWT
for orthopedic nonunion.29 Patients whose nonunion fractures healed
after ESWT treatment had significantly higher serum NO, TGF-β1,
vascular endothelial growth factor (VEGF), and bone morphogenetic
protein 2 (BMP-2) levels after treatment than those with persistent
nonunion.29 Thus, animal work and preliminary human experimental
data points to a complex, multifactorial mechanism of therapeutic
shock waves; however, that ESWT has an effect on biological tissue
based on the current level of scientific knowledge is incontrovertible.
This study demonstrated a clinically important effect of lowenergy defocused shock waves in superficial second-degree burns;
however, the mechanism of action was not studied. Further laser
doppler imaging and burn wound histology, which could have been
illustrative, were not utilized in this study. Although this study is
further limited by modest sample size and lack of long-term followup, the difference in time to complete burn site healing was highly
significant in favor of the shock wave treated group. Statistical significance in favor of shock wave therapy was maintained when imputing
missing data by assuming the worst case scenario for the shock wave
group and the best case scenario for the control group patients with
incomplete data or lost to follow-up. Although not assessed in this
study, quality of life outcome measures should be assessed in future
clinical studies to include assessment of pain, symptom distress, and
profile of mood state over a longer period of follow-up than studied
herein.
Our analyses show that the ESWT advantage persisted when
also controlling for age as a baseline covariate. We chose to perform this additional analysis to simply address the age imbalance between treatment groups. Regarding the study power, we considered a
2-day mean difference between treatments to be clinically meaningful, which could be detected with >80% power for the observed SD
(2 days when pooled across treatment groups). We also note that the
sample size and SD remain adequate after accounting for the loss of
a degree of freedom for including age as a baseline covariate. Thus,
we are confident that the study is adequately powered for a 2-sided
hypothesis test of superiority with a 5% type I error.
CONCLUSIONS
In this randomized phase II clinical trial application of a single
defocused shock wave treatment to the superficial second-degree burn
wound, after debridement, significantly accelerates reepithelialization
at the treated site. The conclusion of ESWT superiority remains when
accounting for a worst case scenario and a baseline age imbalance.
ESWT superiority warrants confirmation in a larger prospective randomized clinical trial. ESWT may prove to be a feasible, noninvasive,
safe, and cost-effective method to enhance the healing of both skin
graft donor sites and superficial second-degree burns.
ACKNOWLEDGMENTS
The authors thank Robin Howard for her significant contribution to this work. We would also like to thank Tiffany Felix for her
invaluable assistance, supported in part by the Henry M. Jackson
Foundation for the Advancement of Military Medicine. We are grateful to the members and staff of the Combat Wound Initiative Program, the AUVA-Trauma Center, and the Unfallkrankenhaus Berlin,
Zentrum fur Schwerbrandverletzte mit Plastischer Chirurgie for their ¨
consistent support of this collaborative research effort.
REFERENCES
- Brown GL, Nanney LB, Griffen J, et al. Enhancement of wound healing by
topical treatment with epidermal growth factor. N Engl J Med. 1989;321:
76–79. - Cribbs RK, Luquette MH, Besner GE. Acceleration of partial-thickness burn
wound healing with topical application of heparin-binding EGF-like growth
factor (HB-EGF). J Burn Care Rehabil. 1998;19:95–101. - Arno A, Garc ´ ´ıa O, Hernan I, et al. Extracorporeal shock waves, a new non- ´
surgical method to treat severe burns. Burns. 2010;36(6):844–849. - Meirer R, Kamelger FS, Piza-Katzer H. Shock wave therapy: an innovative treatment method for partial thickness burns. Burns. 2005;31(7):
921–922. - Ottomann C, Hartmann B, Tyler J, et al. Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock
wave therapy. J Am Coll Surg. 2010;211(3):361–367. - Moher D, Schulz KF, Altman DG. CONSORT. The CONSORT statement:
revised recommendations for improving the quality of reports of parallel group
randomized trials. BMC Med Res Methodol. 2001;1:2. - Ingber DE. Cellular mechnotransduction: putting all the pieces together. FASEB
J. 2006;20:811–827. - Pietramaggiori G, Liu P, Scherer SS, et al. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Ann Surg.
2007;246(5):896–902. - Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–
1096; discussion 1097–1098. - Argenta LC, Morykwas MJ, Marks MW, et al. Vacuum-assisted closure: state
of clinic art. Plast Reconstr Surg. 2006;117(7 Suppl):127S–142S. - Ennis WJ, Formann P, Mozen N, et al. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter
trial. Ostomy Wound Manage. 2005;51:24–39. - Ennis WJ, Lee C, Meneses P. A biochemical approach to wound healing
through the use of modalities. Clin Dermatol. 2007;25:63–72. - Schaden W, Thiele R, Kolpl C, et al. Shock wave therapy for acute and ¨
chronic soft tissue wounds: a feasibility study. J Surg Res. 2007 Nov;143(1):
1–12. - Huemer GM, Meirer R, Gurunluoglu R, et al. Comparison of the effectiveness
of gene therapy with transforming growth factor-beta or extracorporal shock - wave therapy to reduce ischemic necrosis in an epigastric skin flap model in
- rats. Wound Repair Regen. 2005;13:262–268.
- Kuo YR, Wu WS, Hsieh YL, et al. Extracorporeal shock wave enhanced
extended skin flap tissue survival via increase of topical blood perfusion and
associated suppression of tissue pro-inflammation. J Surg Res. 2007;143(2):
385–392. - Aicher A, Heeschen C, Sasaki K, et al. Low-energy shock wave for enhancing
recruitment of endothelial progenitor cells: a new modality to increase efficacy
of cell therapy in chronic hind limb ischemia. Circulation. 2006;114(25):2823–
2830. - Davis TA, Stojadinovic A, Anam K, et al. Extracorporeal shock wave therapy
suppresses the early proinflammatory immune response to a severe cutaneous
burn injury. Int Wound J. 2009 Feb;6(1):11–21. - Stojadinovic A, Elster EA, Anam K, et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis.
2008;11(4):369–368. - Haupt G, Chvapil M. Effect of shock waves on the healing of partial-thickness
wounds in piglets. J Surg Res. 1990;49:45–48. - Meirer R, Kamelger FS, Huemer GM, et al. Extracorporeal shock wave may
enhance skin flap survival in an animal model. Br J Plast Surg. 2005;58:
53–57. - Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave
therapy markedly ameliorates ischemia induced myocardial dysfunction in
pigs in vivo. Circulation. 2004;110:3055–3061. - Fukumoto Y, Ito A, Uwatoku T, et al. Extracoporeal cardiac shockwave therapy ameliorates myocardial ischemia in patients with severe coronary artery
- Arno A, Garcia I, Hernan J, et al. Extracorporal shock waves, a new non-surgical method to treat severe burns. Burns. 2010 Sep;36(6):844–849.
- Gotte G, Amelio E, Russo S, et al. Short-time non-enzymatic nitric oxisynthesis from L-arginine and hydrogen peroxide induced by shock wavestreatment. FEBS Lett. 2002 June 5;520(1–3):153–155.
- Ciampa A, de Prati E, Amelio E, et al. Nitric oxide mediates anti-inflammatory
- action of extracorporeal shock waves. FEBS Lett. 2005 Dec 19;579(30):6839–6845.Mariotto S, Cavalieri E, Amelio E, et al. Extracorporeal shock waves:from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide2005;12:89–96.
- Wang FS, Yang KD, Chen RF, et al. Extracorporeal shockwave promotesgrowth and differentiation of bone-marrow stromal cells towards osteoprogenitors associated with induction of TGF-beta1. J Bone Joint Surg Br.2002;84:457–461.
- Wang FS, Yang KD, Wang CJ, et al. Shockwave stimulates oxygen radicalmediated osteogenesis of the mesenchymal cells from human umbilical cordblood. J Bone Miner Res. 2004 Jun;19(6):973–982.
- Wang CJ, Yang KD, Ko JY, et al. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and BMP-2in long bone non-unions. Nitric Oxide. 2009 Jun;20(4):298–303.
As extracorporeal shock wave therapy (ESWT) can enhance healing of skin graft donor sites, this study focused on shock wave effects in burn wounds.
